-
BASIC DRUG
DISCUSSIONWelcome to Bluelight! Posting Rules Bluelight Rules Benzo Chart Opioids Chart Drug Terms Need Help?? Drugs 101 Brain & Addiction Tired of your habit? Struggling to cope?
Want to regain control or get sober?
Visit our Recovery Support Forums -
BDD Moderators: Keif’ Richards | negrogesic
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
RCs A recup of my discoveries over the past.
- Thread starter Gaffy
- Start date
Here I'll be posting my best inventions, selected for their binding profiles.
It'll take some time to finish this thread (if I ever do as I've litterally thousands of chemicals to sort out and run through, and test with the SAR machine), but I'll post what I think deserves to be posted.
We'll start with Ketamine and Ephenidine analogs I've invented, and switch to BTCP analogs which will permit us to switch to DRIs: (And from there it'll be pretty random, as I can't classify all my compounds)
Ketamine
PCP
Ephenidine
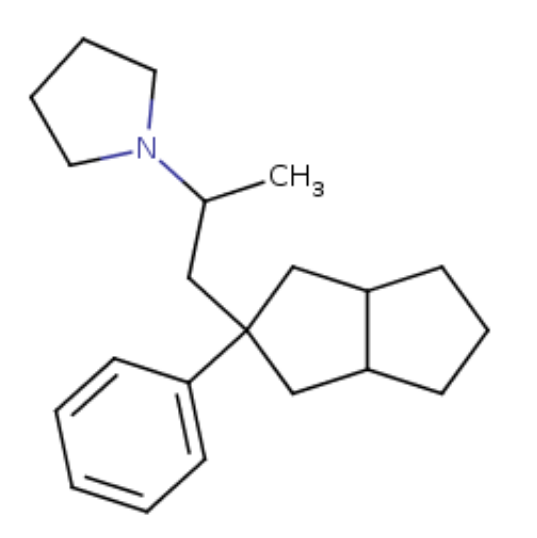
Seven ringed Ketamine,
CycloHeptylKetamine : C1(C(CCCCC1)=O)(C2=CC=CC=C2)N(C)[H]
Seven ringed Methoxetamine,
CycloHeptylMethoxetamine: C1C(C(CCCC1)(C2=CC=CC(=C2)OC)NCC)=O
Adding a benzene group to the cycloheptyl:
3-MeO-PCPy with a PhenylCycloHEptyl ring: C1CC(CCC2=C1C=CC=C2)(C3=CC=CC(=C3)OC)N4CCCC4
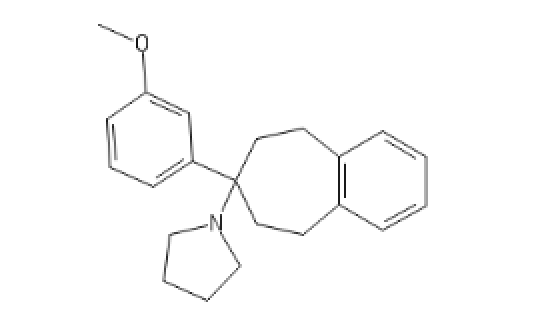
a little extra
(With very interesting SARs)
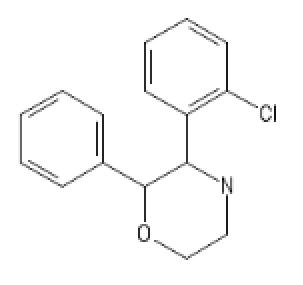
MorphoKetamine: ClC1=C(C=CC=C1)C1NCCOC1C1=CC=CC=C1
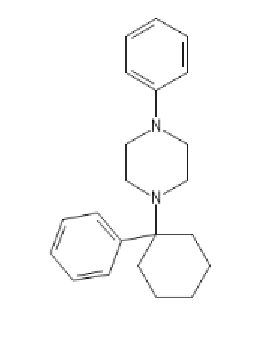
PCP with a N-PhenylPiperazine
PCPP: C1=CC=C(C=C1)N2CCN(CC2)C3(CCCCC3)C4=CC=CC=C4
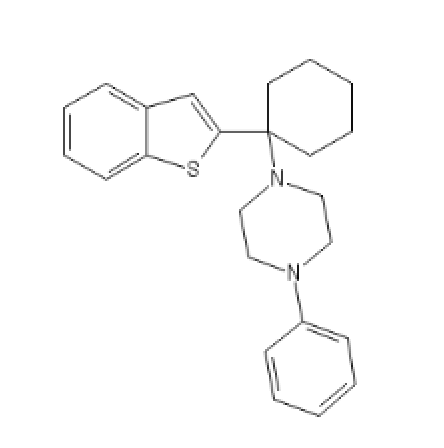
BTCPP, BTCP with a phenylpiperazine
BTCPP: C1=CC=C(C=C1)N2CCN(CC2)C3(CCCCC3)C5=CC4=CC=CC=C4S5
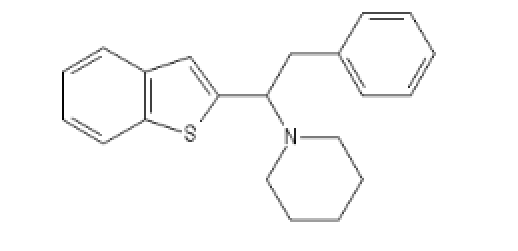
Three analogs between Ephenidine and BTCP:
BTPhenEthylPiperidine : C(C(N1CCCCC1)C1=CC2=C(S1)C=CC=C2)C1=CC=CC=C1
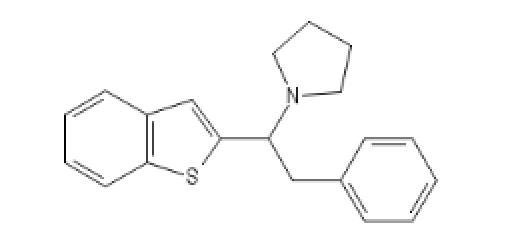
BTPhenEthylPyrrolidine: C12=C(C=CC=C1)SC(=C2)C(CC3=CC=CC=C3)N4CCCC4
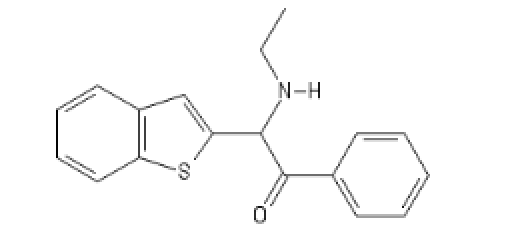
BTBKPhenEthylEthylamine: C12=C(C=CC=C1)SC(=C2)C(C(C3=CC=CC=C3)=O)N(CC)[H]
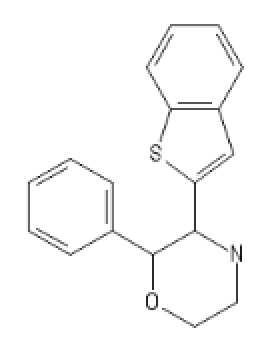
The morpholine analog of ketamine applied to BTCP:
MorphoBTCE: C1COC(C(N1)C1=CC2=C(S1)C=CC=C2)C1=CC=CC=C1
Mono-methylated alpha-phenylated tryptamine, a SNDRI that stands out:
N-Methyl-Alpha-Phenyl-Tryptamine: C1=CC=CC2=C1C(=C[N]2)CC(C3=CC=CC=C3)NC
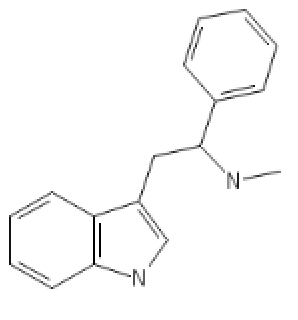
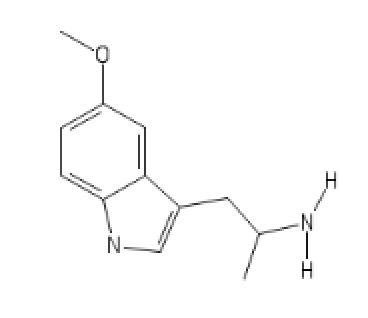
Its N-Pyrrolidino analog
5-MeO-AMT
C1(=CC=C2C(=C1)C(=C[N]2)CC(N([H])[H])C)O
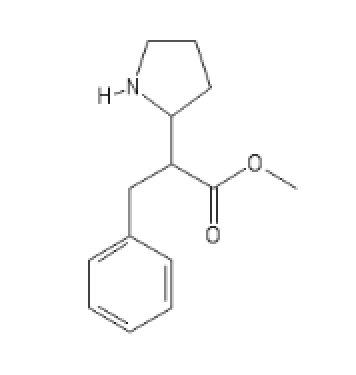
Cocaïne analogs: probably my best inventions
I Don't know what to call these, but they're wonderously promising
COC(=O)C(CC1=CC=CC=C1)C1CCCN1
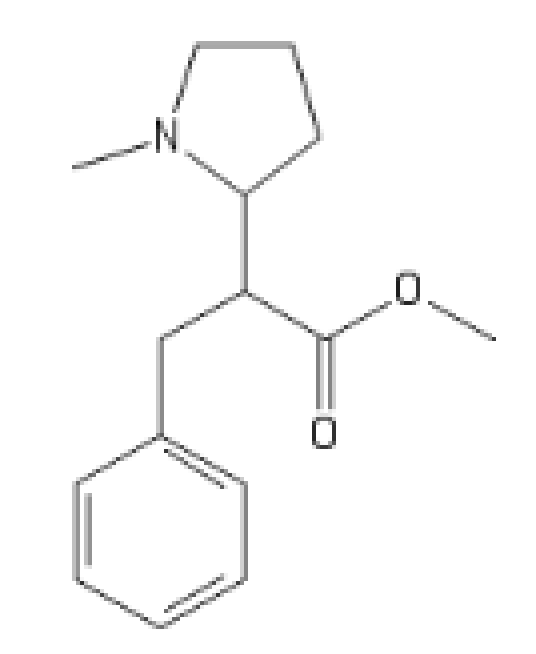
The N-Methyl, even more promising
C1(=CC=CC=C1)CC(C(=O)OC)C2CCCN2C
The local anaesthetic version, it's got high HERG activity:
C1=CC=CC=C1C(OCC(C(OC)=O)C2CCCN2[H])=O
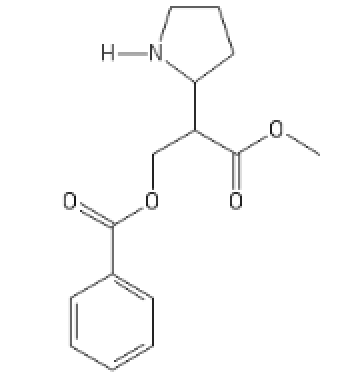
The N-Methyl version, with even more HERG activity, which makes it cardiotoxic
COC(=O)C(COC(=O)C1=CC=CC=C1)C1CCCN1C
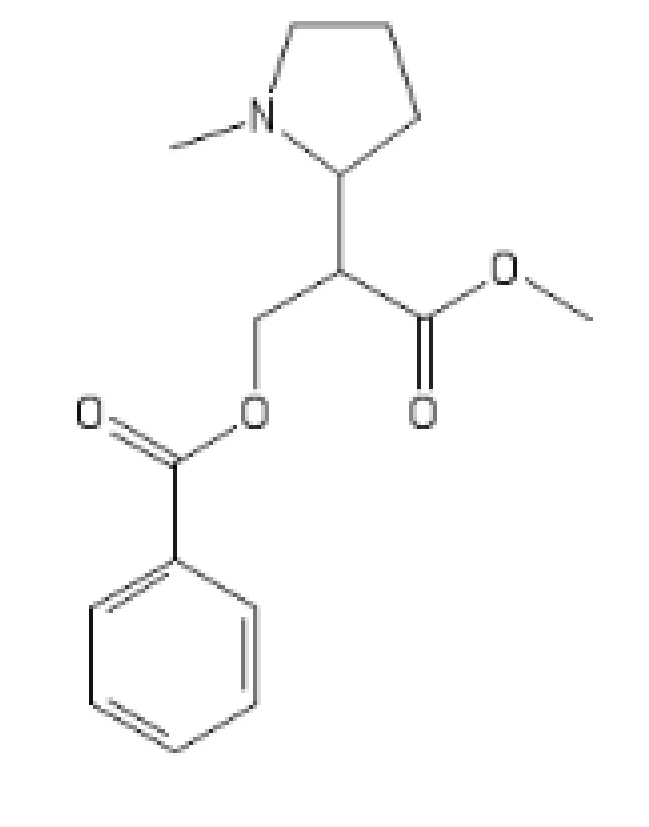
An outstanding one,both SNDRI and MOR (!)
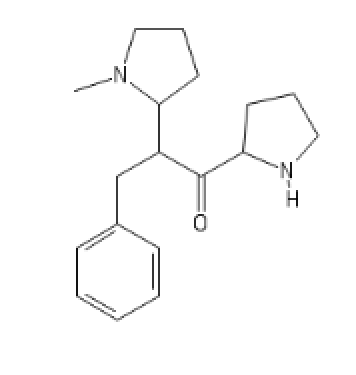
N'-N-Methyl version
2-FA Light
C1(=CC=CC=C1OC2CCN(CC2)[H])F
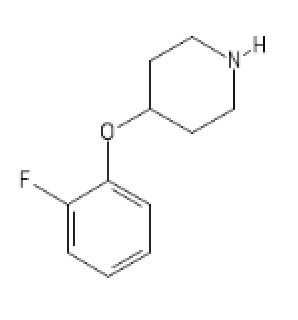
4-MethylMethylphenidate N-pyrrolidine analog
C1=CC=CC=C1C(C(N2CCCC2)=O)C3CCCCN3[H]
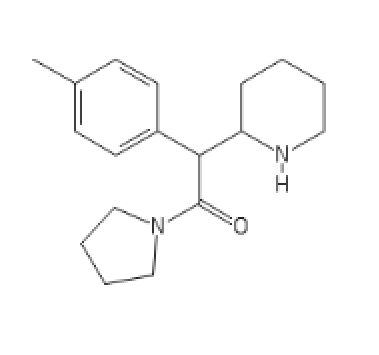
PVP Methylphenidate: Surprisingly good
C1=CC=CC=C1C(C2CCCC3CCCN23)C(OC)=O
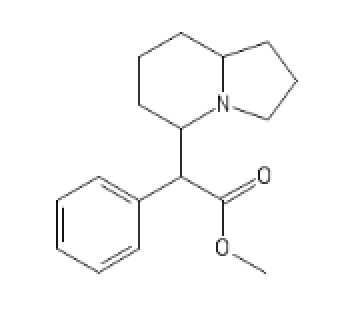
N-pyrrolidino Version
3-MeO-PhenEtrazine
C1=C(C=CC=C1C2C(CC)N(CCO2)[H])OC
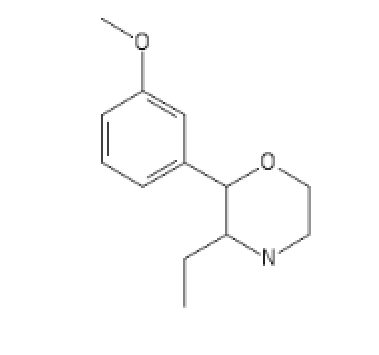
CyclopentylEthylCathinone
CCNC(C1CCCC1)C(=O)C1=CC=CC=C1
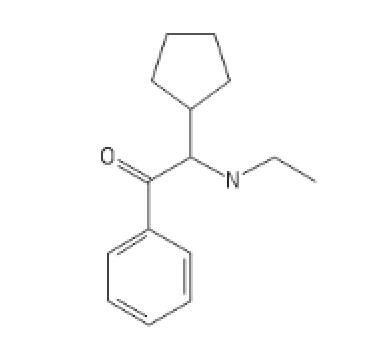
Two Ketobemidone variants, go guess how I guessed
BTKETO1
C1=CC=C2C(=C1)C=C(S2)C3(CCN(CC3)C)CN4CCCC4
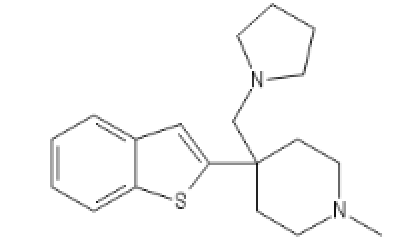
BTKETO2
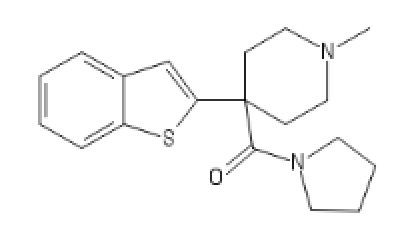
C1=CC=C2C(=C1)C=C(S2)C3(CCN(CC3)C)C(N4CCCC4)=O
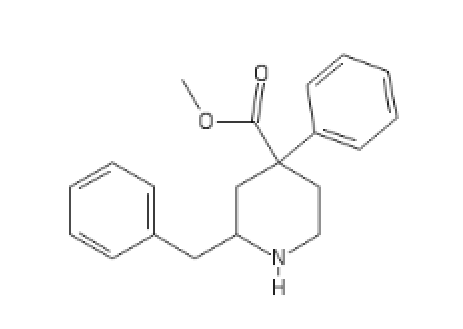
Should be opioïd, is Dopaminergic and Opioïd sigma active:
C2(CC(CC1=CC=CC=C1)N(CC2)[H])(C(=O)CC)C3=CC=CC=C3
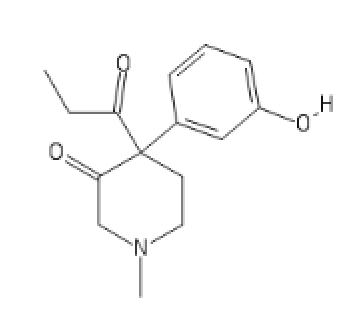
Ketobemidone analog:
C1(C(CN(CC1)C)=O)(C(=O)CC)C2=CC(=CC=C2)O[H]
Chlorphenamine derived Antihistaminergic Psychedelics, or chillaxed tripping!
5-Meo one
C1=C(C=CC2=C1C(=C3C=CC=C[N]23)CCN(C)C)O
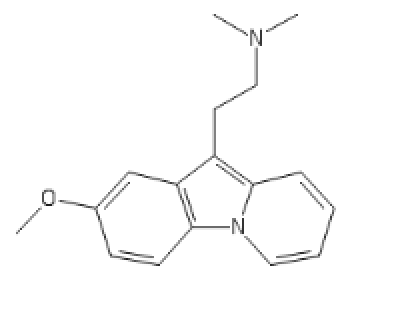
Chlorphenamine inspired
C1=CC(=CC2=C1C(=C3C=CC=C[N]23)CCN(C)C)Cl
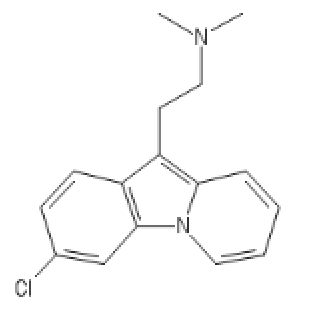
5-Meo DIPT one
C1=C(C=CC2=C1C(=C3C=CC=
I meant the picture I committed to naming GAFFY in the Name-A-Molecule thread ^^ This one:
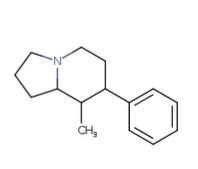
A-AVP
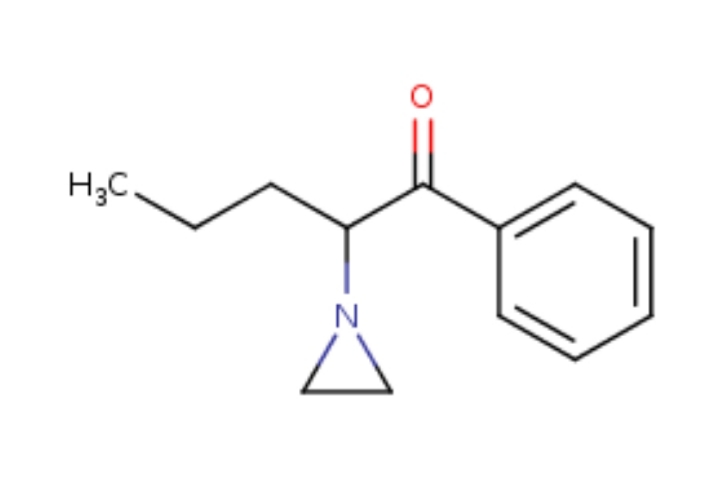
Alpha-AziridineValeroPhenone
Edit:I just realised the difference between pyrrolydine and pyridine.. I will update that asap!
Done
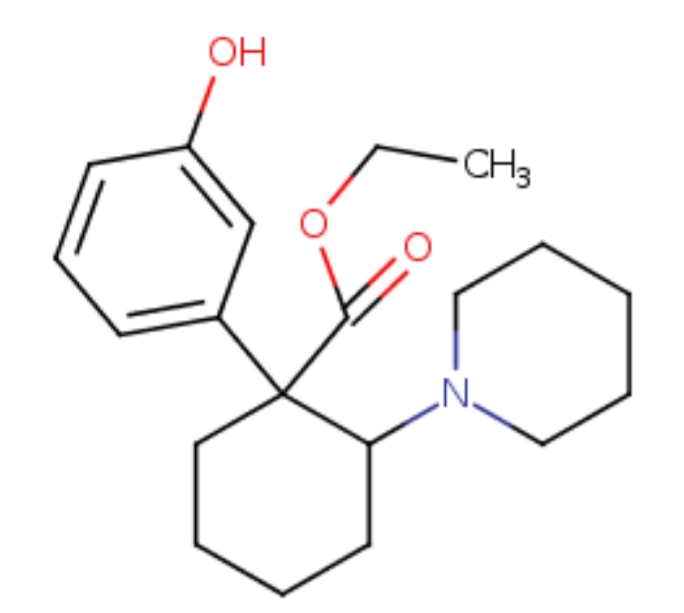
Ethyl 2-(Piperidino)-1-3'-hydroxyphenylcyclohex-3-ene-1-carboxylate
I also like this one. Can't post STP results but it seems lile a winner.
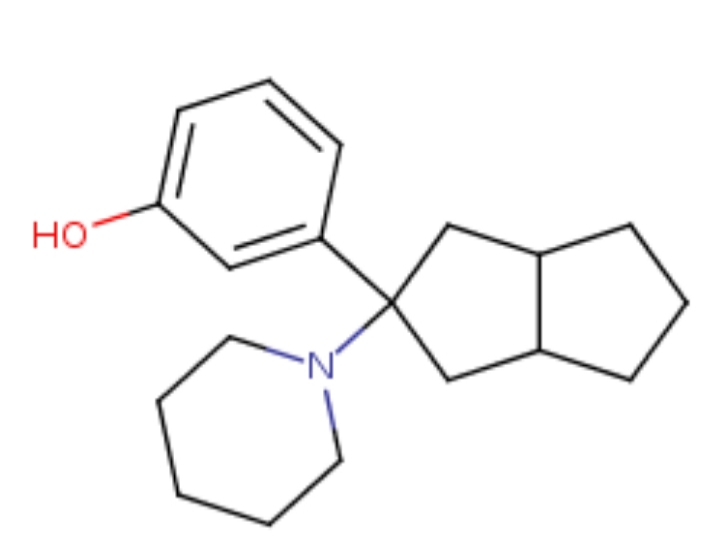
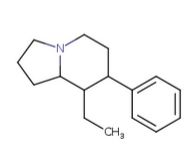
8-Ethyl-7-PHenYLoctahydrOIndoliziNE
EPHYLONE
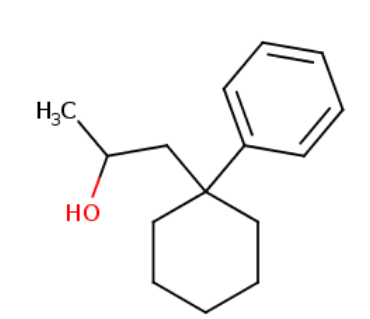
Dopaminergic cannabinoid, shares androgen and estrogen liability. Should be euphoric.
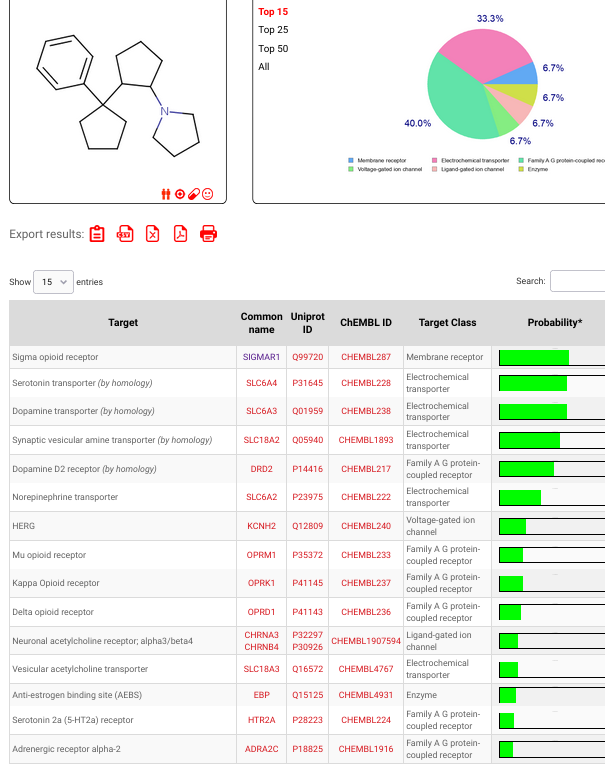
Now let'd take the above and make it a bit more expensive, to get pretty much similar effects.. As of yet, a gold holding dopamine agonist etc etcSwissTargetPrediction
swisstargetprediction.ch
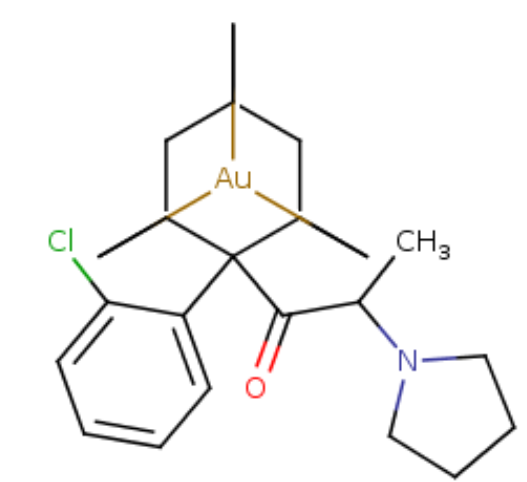
Just a little example of the many possibilitys