-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
Harmane seems to be the most LSD-like of all harmala alkaloids. Unfortunately it's only a minor component in peganum harmala and probably difficult to separate.

 www.sciencedirect.com
www.sciencedirect.com

A comparison of N,N-dimethyltryptamine, harmaline, and selected congeners in rats trained with LSD as a discriminative stimulus
1.1. A series of N-substituted tryptamines was compared with a series of beta-carbolines in rats trained to discriminate LSD (0.1 mg/kg) from saline.2…
1. A series of N-substituted tryptamines was compared with a series of beta-carbolines in rats trained to discriminate LSD (0.1 mg/kg) from saline.
2. Intermediate levels of substitution were elicited by MDMT (76.4% ), DMT (77.9% ), and DET (48.7% ). 6-F-DET produced 41.3% LSD-appropriate responding at a dose of 6.0 mg/kg but only 4 of 8 subjects completed the test session thus precluding statistical analysis. Bufotenine (25.8% ) also failed to substitute. Although none of the tryptamines substituted completely for LSD, the pattern of substitution is consonant with what is known of their activity in humans. MDMT, DMT, and DET are well established in the literature as hallucinogens but the same cannot be said for 6-F-DET and bufotenine.
3. Of the beta-carbolines tested, none substituted for LSD completely and only harmane elicited intermediate substitution (49.5% ). No significant generalization of the LSD stimulus to 6-methoxyharmalan, harmaline, or THBC was observed. Thus, in contrast to the tryptamines, scant ability to substitute for LSD was observed in the beta-carbolines tested.
4. Taken together, the present data indicate that the representative tryptamines employed in the present study exhibit greater similarity to the LSD stimulus than do representative beta-carbolines. The receptor interactions responsible for these differences remain to be determined.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,448
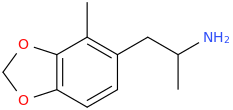
DOROTHY
1-(2-methyl-3,4-methylenedioxyphenyl)-2-aminopropane
Those Who Do Not Learn From Their Mistakes In Their Past Are Doomed To Repeat It.
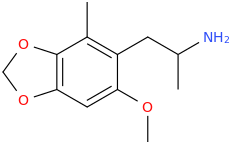
TODO
1-(2-methyl-3,4-methylenedioxy-6-methoxyphenyl)-2-aminopropane
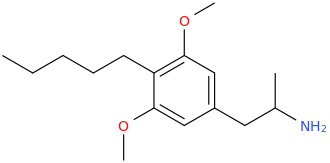
VALERO
1-(4-amyl-3,5-dimethoxyphenyl)-2-aminopropane
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,448
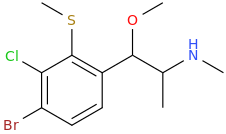
CAMPBELL'S
1-(2-methylthio-3-chloro-4-bromophenyl)-1-methoxy-2-methylaminopropane
If the N-methyl isn't active, the primary amine will be.
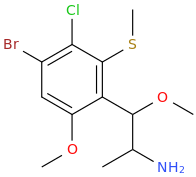
LUIGI'S ALPHABET SOUP
1-(2-methylthio-3-chloro-4-bromo-6-methoxyphenyl)-1-methoxy-2-aminopropane
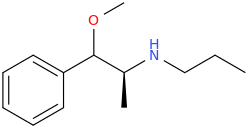
PROFUSALEH
(2S)-1-phenyl-1-methoxy-2-propylaminopropane
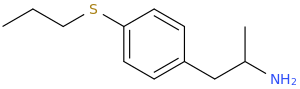
STEWIE
1-(4-propylthiophenyl)-2-aminopropane
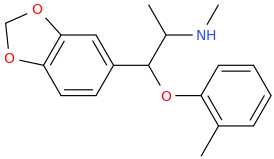
MIAMI
(1-(3,4-methylenedioxyphenyl)-2-methylaminoprop-1-yl)-(2-methylphenyl)-ether
![(2S,5R,6R)-3-(%7B2-[(iminoethyl)amino]ethyl%7Dthio)-2-carbomethoxy-4-mercura-6-(%7B[3-phenyl-3-(3,4-methylenedioxyphenyl)-prop-2-yl]amino%7D)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F%282S%2C5R%2C6R%29-3-%28%257B2-%5B%28iminoethyl%29amino%5Dethyl%257Dthio%29-2-carbomethoxy-4-mercura-6-%28%257B%5B3-phenyl-3-%283%2C4-methylenedioxyphenyl%29-prop-2-yl%5Damino%257D%29-7-oxo-1-azabicyclo%5B3.2.0%5Dhept-2-ene.png&hash=02f462e1f777bded5f970cd1d47e0ad7)
THE MAD HATTER
(2S,5R,6R)-3-({2-[(iminoethyl)amino]ethyl}thio)-2-carbomethoxy-4-mercura-6-([3-phenyl-3-(3,4-methylenedioxyphenyl)-prop-2-yl]amino)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene
antibiotic
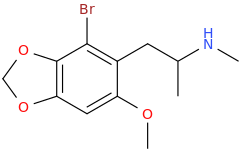
MADONNA
1-(2-bromo-3,4-methylenedioxy-6-methoxyphenyl)-2-methylaminopropane
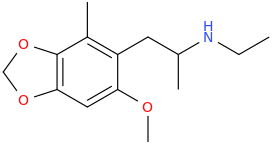
LOURDES
1-(2-methyl-3,4-methylenedioxy-6-methoxyphenyl)-2-ethylaminopropane
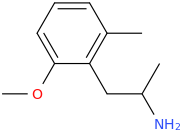
SYFY
1-(2-methyl-6-methoxyphenyl)-2-aminopropane
Last edited:
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
Not quite neuroscience, or pharmacology, but it does use organic chemistry. The relationship to learning systems in computation will likely have lots of crossover to neuroscience, especially as they work on these both by the methods of augmenting molecular configurations:
‘Designer molecules’ could create tailor-made quantum devices
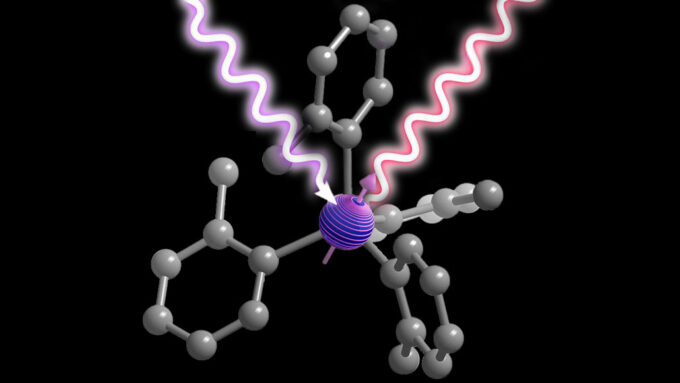
“A molecule with a central chromium ion (purple) can serve as a quantum bit, encoding information in the direction of its spin (indicated by its arrow in this illustration). Attached atoms (gray) alter the properties of the ion, allowing it to be manipulated by a laser (purple squiggle) and to emit light in response (red squiggle).”
‘Designer molecules’ could create tailor-made quantum devices

“A molecule with a central chromium ion (purple) can serve as a quantum bit, encoding information in the direction of its spin (indicated by its arrow in this illustration). Attached atoms (gray) alter the properties of the ion, allowing it to be manipulated by a laser (purple squiggle) and to emit light in response (red squiggle).”
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,448
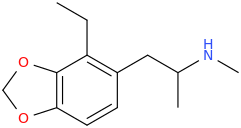
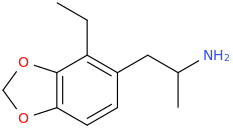
EMERIL'S EMERALD
1-(2-ethyl-3,4-methylenedioxyphenyl)-2-methylaminopropane
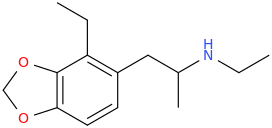
NICOLE MILLER
1-(2-ethyl-3,4-methylenedioxyphenyl)-2-ethylaminopropane
These May Or May Not Be Active.
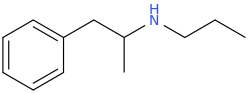
JERRY JERICHO
1-phenyl-2-propylaminopropane
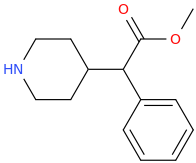
ETON
1-phenyl-1-carbomethoxy-1-(piperidine-4-yl)methane
What Does It Do?
I Don't Know. What Do You Want It To Do?
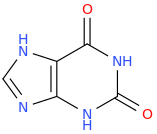
HAF CAF (xanthine)
3,7-dihydro-purine-2,6-dione
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,448
Did I Lie To You?
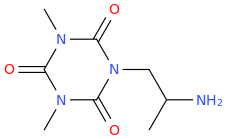
MAGIC POISON
1-(2,4,6-trioxo-1,3,5-triaza-3,5-dimethylcyclohexane-1-yl)-2-aminopropane
Life Of
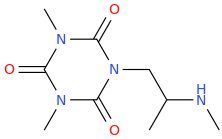
BRIAN
1-(2,4,6-trioxo-1,3,5-triaza-3,5-dimethylcyclohexane-1-yl)-2-(methylamino)propane
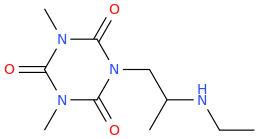
PALLAS 'SCARLET' ATHENA de Swainsboro
1-(2,4,6-trioxo-1,3,5-triaza-3,5-dimethylcyclohexane-1-yl)-2-(ethylamino)propane
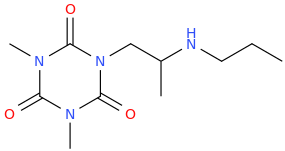
CHRISTOPHER 'DINO' DANIEL BOWEN
1-(2,4,6-trioxo-1,3,5-triaza-3,5-dimethylcyclohexane-1-yl)-2-(propylamino)propane
Bon apetit!
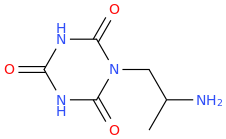
MONA
1-(2,4,6-trioxo-1,3,5-triazacyclohexane-1-yl)-2-aminopropane

MAGIC POISON
1-(2,4,6-trioxo-1,3,5-triaza-3,5-dimethylcyclohexane-1-yl)-2-aminopropane
Life Of

BRIAN
1-(2,4,6-trioxo-1,3,5-triaza-3,5-dimethylcyclohexane-1-yl)-2-(methylamino)propane

PALLAS 'SCARLET' ATHENA de Swainsboro
1-(2,4,6-trioxo-1,3,5-triaza-3,5-dimethylcyclohexane-1-yl)-2-(ethylamino)propane

CHRISTOPHER 'DINO' DANIEL BOWEN
1-(2,4,6-trioxo-1,3,5-triaza-3,5-dimethylcyclohexane-1-yl)-2-(propylamino)propane
Bon apetit!

MONA
1-(2,4,6-trioxo-1,3,5-triazacyclohexane-1-yl)-2-aminopropane
Last edited:
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
I don't see how I could pose the question any simpler: If there was a xanthine whose metabolite in the human body by any pathway was uric acid for the molecule in question (the xanthine), what would you name such a compound. ;-j (it was a question posed to everyone though, not you alone, but any answer would suffice for me ;-j )I'm not sure I understand your question.
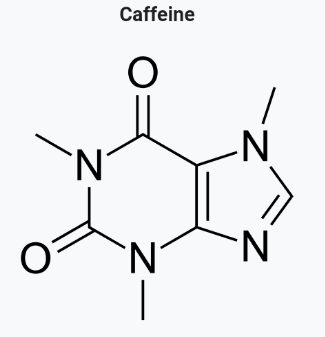
Uric acid (urine):

Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
Out of all possible configurations of a xanthine, when a simple methylene unit added will do?Not metabolically likely at all.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,448
Methylene is R-CH2-R', where R stands for radical.
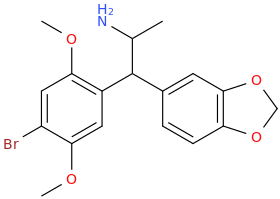
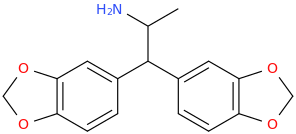
ROEROE
1-(4-bromo-2,5-dimethoxyphenyl)-1-(3,4-methylenedioxyphenyl)-2-aminopropane
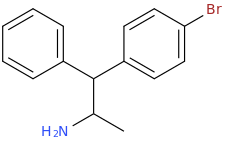
HOPE
1-phenyl-1-(4-bromophenyl)-2-aminopropane
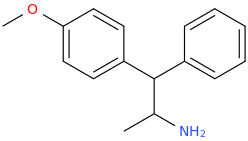
CHAD
1-(4-methoxyphenyl)-1-phenyl-2-aminopropane
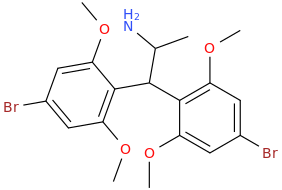
TROY
1,1-bis-(2,6-dimethoxy-4-bromophenyl)-2-aminopropane

ROEROE
1-(4-bromo-2,5-dimethoxyphenyl)-1-(3,4-methylenedioxyphenyl)-2-aminopropane

HOPE
1-phenyl-1-(4-bromophenyl)-2-aminopropane

CHAD
1-(4-methoxyphenyl)-1-phenyl-2-aminopropane

TROY
1,1-bis-(2,6-dimethoxy-4-bromophenyl)-2-aminopropane
Last edited:
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
I meant any substitution coming off the nitrogen that eventually ended in one, not straight off of it.Methylene is R-CH2-R', where R stands for radical
Regardless, I wasn't trying to get in a debate about the viability, and which is why I did not limit the scope and present as example one specific instance of any sort, partly due to knowing that making it active would be a chore. I just thought the concept would make for a witty, facetious, name.
Namely (pun intended) It was a purposely abstract and flippant (much as this whole thread) request, not an invitation for analysis. ;-P I can't help but feel this thread needs more substance, setting any kind of precedent in that regard was my intention, broaden the spectrum of content a bit with some interaction, have something more than a series of stand alone posts.