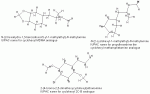
1-(3,4-methylenedioxycyclohexyl)-2-methylaminopropane better naming -thx
The above chemical would be a cyclohexane version of MDMA. Shorter name 3,4-methylenedioxy-propylhexedrine, if you will. Maybe call it MDPH. Has this chemical ever been proposed, discussed, synthesized, or tasted??
I would imagine the task of building a methylenedioxy ring onto a boat or a chair shape would be very difficult. My thoughts are that if synthesis is possible then it may have some mdma qualities, in much the way that propylhexedrine approximates some of methamphetamine's qualities.
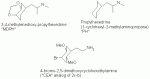
Would it be possible to create the 3,4-methylenedioxy with a cyclohexadiene form (as from hexatriene). Why is every drawing of Propylhexedrine that I can find represented as planar (like benzene)? There are no double bonds as it is cyclohexane, therefore not planar, and chair or boat shaped (planar cyclohexane would be the highest energy state possible). Am I wrong or is every structural representation i've seen of this chemical.
Maybe the loss of potency would be so great as to not be usable. Propylhexedrine is a far less potent than than methamphetamine. Could it be that the dosage needed of 'MDPH' for entactogenic experience would be so high that the body load or peripheral effects would be too much for a person to take?
Would it be possible then to set about making cyclohexyl versions of all PEA (phenethylamines) creating new CEAs (cyclohexethylamines). I would imagine that all of these would have much reduced potency if they indeed had any, but might this not be a good way to circumnavigate analogue laws? because anyone with a background in chemistry can tell you that the shape of cyclohexane is nothing like a phenyl ring, possibly making the analogue argument a bust.
I'm sure there are issues which i am not taking into much account about how unstable the structural shape of cyclohexane is compared to benzene. Maybe one day i'll do all this and write the CIHKAL. If cyclohexane was used there would be some wild looking 3d structures.
Does anyone have any info about propylhexedrine trials or studies? Any info on binding affinities and such? I would really like see data about how propylhexedrine compares to methamphetamine. Anything from adrenergenic activity to release of DA, NE, and SERT.
And now, please tell me what you think.
thanks
-DT
The above chemical would be a cyclohexane version of MDMA. Shorter name 3,4-methylenedioxy-propylhexedrine, if you will. Maybe call it MDPH. Has this chemical ever been proposed, discussed, synthesized, or tasted??
I would imagine the task of building a methylenedioxy ring onto a boat or a chair shape would be very difficult. My thoughts are that if synthesis is possible then it may have some mdma qualities, in much the way that propylhexedrine approximates some of methamphetamine's qualities.
Would it be possible to create the 3,4-methylenedioxy with a cyclohexadiene form (as from hexatriene). Why is every drawing of Propylhexedrine that I can find represented as planar (like benzene)? There are no double bonds as it is cyclohexane, therefore not planar, and chair or boat shaped (planar cyclohexane would be the highest energy state possible). Am I wrong or is every structural representation i've seen of this chemical.
Maybe the loss of potency would be so great as to not be usable. Propylhexedrine is a far less potent than than methamphetamine. Could it be that the dosage needed of 'MDPH' for entactogenic experience would be so high that the body load or peripheral effects would be too much for a person to take?
Would it be possible then to set about making cyclohexyl versions of all PEA (phenethylamines) creating new CEAs (cyclohexethylamines). I would imagine that all of these would have much reduced potency if they indeed had any, but might this not be a good way to circumnavigate analogue laws? because anyone with a background in chemistry can tell you that the shape of cyclohexane is nothing like a phenyl ring, possibly making the analogue argument a bust.
I'm sure there are issues which i am not taking into much account about how unstable the structural shape of cyclohexane is compared to benzene. Maybe one day i'll do all this and write the CIHKAL. If cyclohexane was used there would be some wild looking 3d structures.
Does anyone have any info about propylhexedrine trials or studies? Any info on binding affinities and such? I would really like see data about how propylhexedrine compares to methamphetamine. Anything from adrenergenic activity to release of DA, NE, and SERT.
And now, please tell me what you think.
thanks
-DT
Last edited: