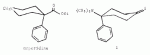Undetailed
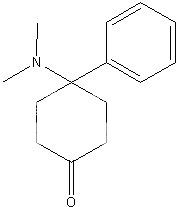
Actually, the horrifying thing is, this molecule is ridiculously easy to synthesize. Here is a synthesis that would only be understood by chemists and is not a "threat" of giving anything to anybody.I think this should be sufficiently cryptic enough, not to go against our "no synthesis" rule
!) protected cyclohexanedione, 4-Br-phenyl Grignard (yes, it exists). Barbier type reaction with appropriate imine.
2) Deprotection
3 Obvious Grignard
I would hate to see anyone ever do it and put it one the street, just contemplating anything that toxic makes me queasy. The formulation would be bound to go wrong in the hands of amateurs, and result in many deaths.
Smyth said:It's the trans isomer that is the most active, and the synth is not what I would call "easy" by any stretch.
Actually, the horrifying thing is, this molecule is ridiculously easy to synthesize. Here is a synthesis that would only be understood by chemists and is not a "threat" of giving anything to anybody.I think this should be sufficiently cryptic enough, not to go against our "no synthesis" rule
!) protected cyclohexanedione, 4-Br-phenyl Grignard (yes, it exists). Barbier type reaction with appropriate imine.
2) Deprotection
3 Obvious Grignard
I would hate to see anyone ever do it and put it one the street, just contemplating anything that toxic makes me queasy. The formulation would be bound to go wrong in the hands of amateurs, and result in many deaths.
Last edited:




 ), although to be honest, Tony Blair had already started to look like he was well on the way to a helter-skelter moment, this was his opportunity to be remembered (a wiser person might have just been happy with the Northern Ireland peace agreement, but nooooo...)
), although to be honest, Tony Blair had already started to look like he was well on the way to a helter-skelter moment, this was his opportunity to be remembered (a wiser person might have just been happy with the Northern Ireland peace agreement, but nooooo...)