sekio
Bluelight Crew
Water and methanol are pretty different in terms of dissolving power. I wouldn't substitute anything; methanol is easy to acquire most places and Hofmann would have used water if it had worked, I'm sure - it costs less.
N&PD Moderators: Skorpio | someguyontheinternet
Water and methanol are pretty different in terms of dissolving power. I wouldn't substitute anything; methanol is easy to acquire most places and Hofmann would have used water if it had worked, I'm sure - it costs less.
 screen shot
screen shotI'm guessing there's a "destruction point", which is something a fair bit greater than the melting point? Does anyone know the melting points of ergine and ergonovine?
Interesting. I had always heard that Riveas were stronger, but after some searching it seems the seeds are just bigger.Rivea corymbosa consisted of 0.01% alkaloids, and Ipomoea violacea consisted of 0.05% alkaloids.
The spectrum of alkaloids present proved to be almost identical for both samples.
I have doubts you can "boil" ergot alkaloids!
Your doubts would seem to be well founded, I had no idea such a respected software suite would be defaulting to probability theory without some kind of warning being attached to the analysis of the molecule.

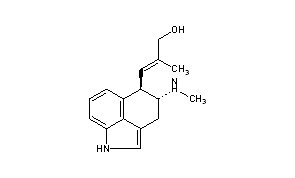
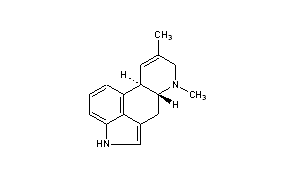
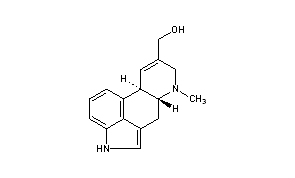
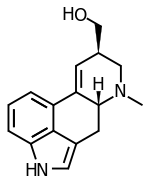
................................... Is anyone aware of sources for the properties of the ergoline alkaloids found in morning glory seeds? This would provide insight as to the cleanness of different types of extractions.
aren't these properties what we're taking advantage of to narrow the targets for extraction. Whats wrong with going with simplicity like acetone with a mild acid, citric acid/acetone or tartaric acid in methanol with a NP solvent rinse.

Can you elavorate? What I had in mind was separating the clavine alkaloids from the lysergamides. Webster's literature says chanoclavine, one of the clavines, is insoluble in water (sekio's chanoclavine link confirms this). So I was thinking even a simplistic kitchen preparation would be able to eliminate this one ergoline -- and more, assuming there are other clavines that are poorly soluble in water.................