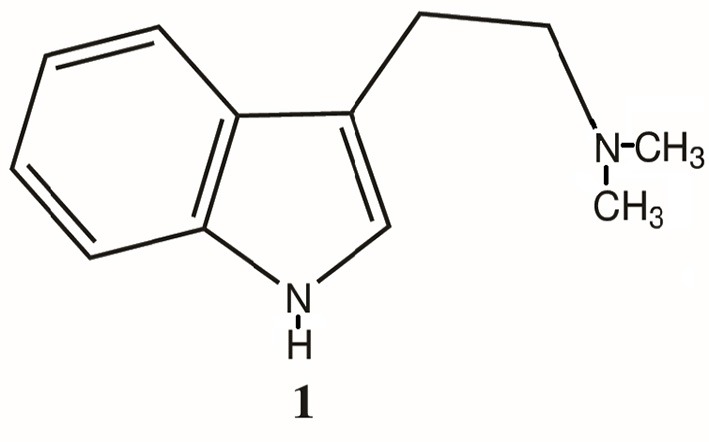
mr peabody
Bluelight Crew
An explanation of the effect of psychedelics on the nervous system at the level of the neurone
This article is intended to be a detailed explanation of how hallucinogens affect the brain, via the inhibition and excitation of neurotransmitters in the nervous system. This article is not written by me, but an extremely close friend of mine who is significantly more knowledgeable when it comes to science, and specifically chemistry.
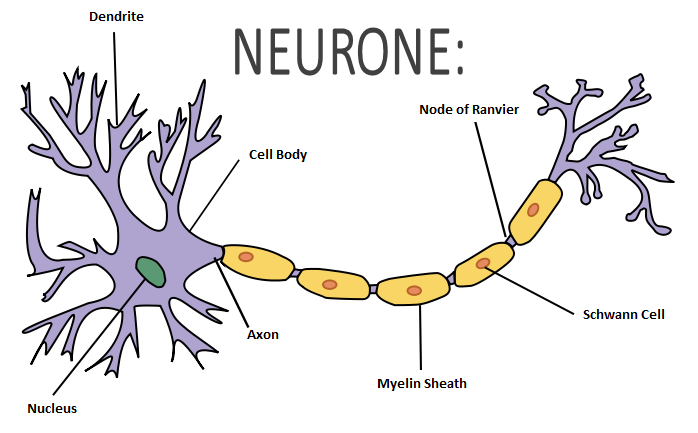
A neurone is a cell that carries electrical impulses. Information is processed and transmitted by the nervous system in the form of electrical and chemical signals. The neurone primarily comprises of a cell body, an axon and dendrites. The axon is a nerve fibre that carries electrical signals away from the cell body, to the end of the neurone. The end of the neurone then connects to the dendrites on the cell body of the next neurone, via a synapse. Drugs cause their effects due to their action on the neurones in the nervous system.
A nerve impulse is a self-propagating wave of electrical disturbance that travels along the surface of the axon membrane. This electrical disturbance comprises of a temporary reversal of the electrical potential difference; not an electrical current. The axon is usually negatively charged compared to the outside of the axon, and this is known as the resting potential, the value of which is usually around -65mV. When a stimulus is received, a reversal in electrical potential difference is caused, and this is known as the action potential (normally around +40mV).
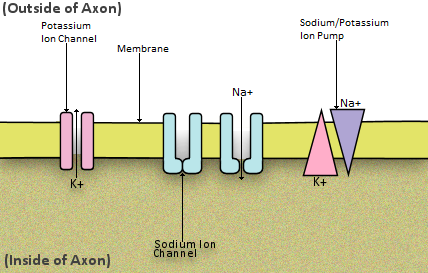
To begin, the inside of the axon is negatively charged, compared with the outside of the axon. The change in potential difference, that is needed to fire off an action potential, is controlled by the movement of sodium and potassium ions in and out of the axon. An ion is a positively or negatively charged molecule. This movement occurs via the action of ion pumps and channels. The ions cannot just diffuse in and out of the axon uncontrollably; this diffusion is prevented by a membrane around the axon. Periodically placed along the membrane are proteins that act as channels for ions to pass through. Sodium gated channels and potassium gated channels open and close to allow the ions to pass through only at specific times. Sodium-potassium pumps transport Na+ and K+ in and out of the axon.
The inside of the axon starts at around -65mV compared to the outside of the axon. An action potential is reached when the axon is at +40mV compared to the outside of the axon. This value of +40mV is reached by the movement of sodium and potassium ions in and out of the axon. Sodium-potassium pumps transport 2K+ into the axon for every 3Na+ transported out of the axon. Both sodium and potassium are in the forms of positive ions here. However, more sodium is removed from the axon compared to the potassium brought. This means the overall electro negativity is decreasing in the axon, and the axon is getting closer to reaching the potential difference of +40mV. Sodium ions then begin to diffuse back into the axon naturally, and potassium ions diffuse back out. At this stage however, potassium gated channels are open, whereas sodium gated channels are closed. This means the K+ can diffuse out faster than the Na+ can diffuse back into the axon. This increases the potential difference further between the inside and the outside of the axon.
Read the full article:
http://disregardeverythingisay.com/p...ucinogens-upon
Last edited by a moderator: