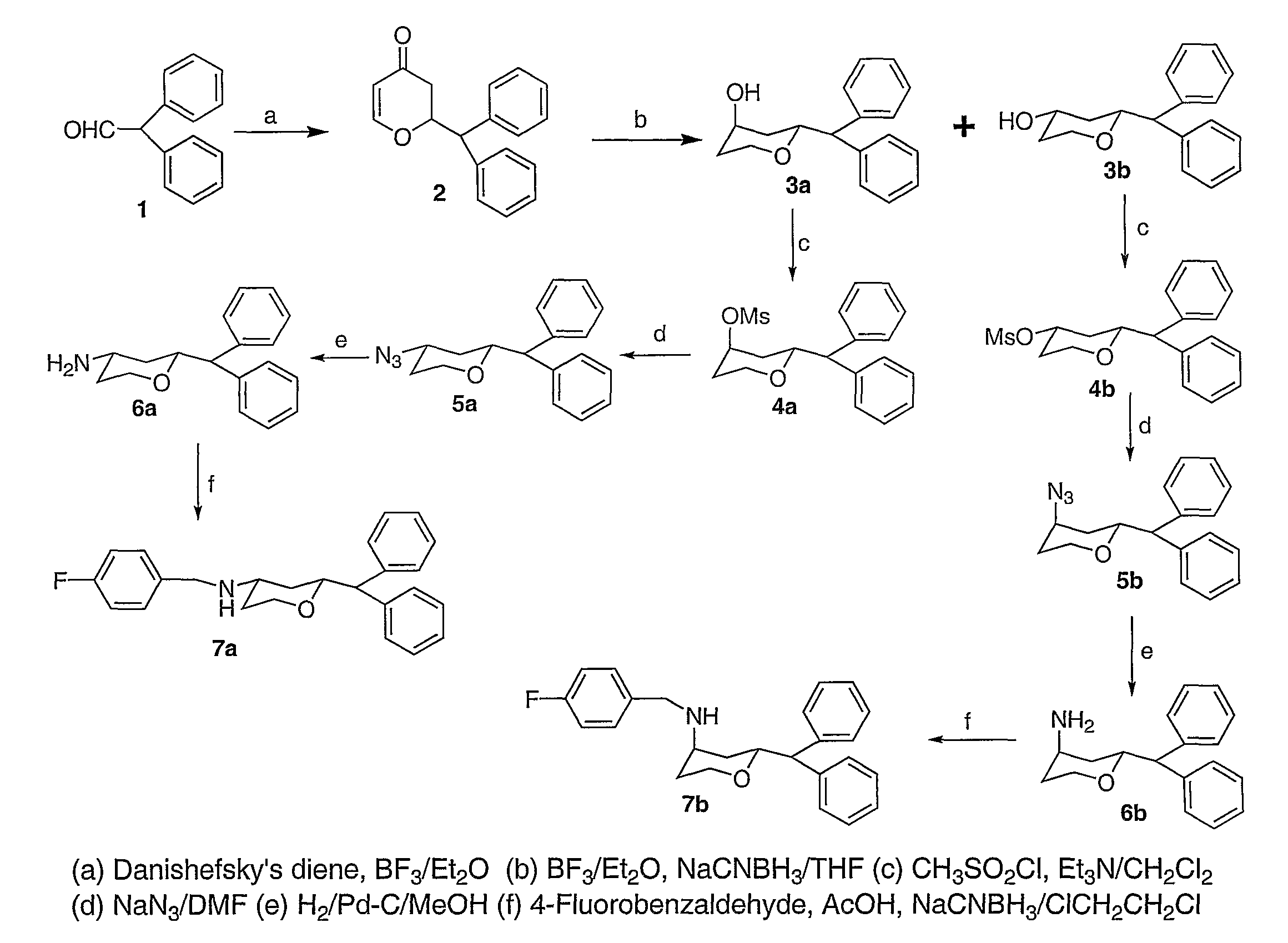
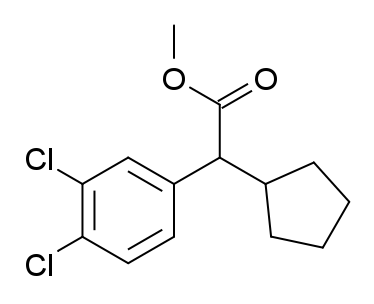
Yeah, I meant the thioester, and just thinking of something that liberated methaneselenol in-vivo is bad enough.
Having one's body turned into a weapons-grade stink bomb factory isn't a pleasant experience. Never had the..well I wouldn't use the term 'pleasure', with selenium or Te, but sulfurous nasties of that general flavour (quite literally, when it also comes out in one's saliva and nasal mucus, along with pretty much any, and for that matter EVERY possible kind of bodily leavings or loose parts), and after a week of that, you'll never want to smell volatile mercaptanesque substances again for the remainder of your natural lifespan, if for some strange reason, anybody would have wanted to to begin with

And agreed about ethers, I'd expect them to undergo oxidative metabolism rather than cleavage.
As for Ac-CoA, well don't forget to take into account, in that specific context, just how specifically tuned they are to act as in essence, surgically precise and pinpoint-accurate, high-efficiency instruments, with hydrogen bonding, electrostatic considerations, steric constraints, solvent shell effects, potentially halogen bonding, to make them very good at performing their specific tasks, generally better than we can accomplish with traditional wet-chem, no?
Just look at phytochemistry/mycochemistry, and how with a few enzymes and basic substrates to start with, plants, fungi can produce the likes of morphine, galantamine (which coincidentally have quite a lot in common, indeed one can do, if one were to be so inclined and have the competency, so much as to begin a total sythesis, and have it diverge so as to use a common precursor to either to produce both), alkaloids like strychnine, or in the fungal case, the ergoloids from dimethylallyl pyrophosphate. They do in very mild conditions, what would take a human chemist a staggering amount of effort in comparison, and a long bloody time to do it in too.
So perhaps an enzymatically mediated process isn't the best case for illustrating stability, relatively speaking.
And who the fuck would think to include tellurium in a drug? potential for toxicity aside, Te is notorious for the unspeakable stench given off by people who have intaken only small quantities of the element, as 'tellurium breath', which from everything I've read, sounds like it affects bodily off-castings in general rather than specifically the breath, kind of similar sounding to my unfortunate sulfurous experience of a few years back, only much, much worse.