JohnBoy2000
Bluelighter
- Joined
- May 11, 2016
- Messages
- 2,463
This may sound like a far reaching theory but - I'm a firm believer in patterns.
Patterns of the universe, mathematical patterns, behavioral patterns etc - Fibonacci series is the one we're probably most familiar with.
Mathematicians have long since been attempting to determine patterns in the stock market.
Again - there's specifically no scientific data to document this in relation to pharmacology but - I've been using a combination of Atomoxetine and Mianserin to provide the most potent noradrenergic boost.
Subsequently - I have been attempting to introduce a serotonergic component via an SSRI/NRI type of drug - with limited success.
This is mainly due to their poor ability to combine well with the former two.
As Mianserin and mirtazapine basically have the same interaction profile - I thus attribute it to poor interaction potential with Atomoxetine - as SSRI/NRI's are well documented in combination with mirtazapine.
The only two large scale trials on Atomoxetine in combination has been with Sertraline, and fluoxetine.
I have attempted;
- Venlafaxine
- Escitalopram
- Sertraline
- Duloxetine
- Desvenlafaxine
- Brintellix
... as potential agents.
The closest I came was with Sertaline - and to a far lesser extent, Duloxetine.
I selected these on the basis of their CYP inhibition profiles (being low), as Atomoxetine is highly sensitive to CYP2D6 inhibition.
However - a very prevalent side effect - was excessive sweating.
This indicates to me, a highly taxed central nervous system.
Additionally - over the course of 24 hours - the best results were always had dosing, in between Atomoxetine and Mianserin - as I would normally dose these about 8:00, and 22:00.
So somewhere around 16:00 for the third agent.
This would suggest sufficient metabolic time for each agent.
My most recent attempt - has been with Fluoxetine.
I selected this as, looking for some kind of symmetry or pattern relative to the other two drugs - which I had previously sought in terms of the pharmacological profiles - unsuccessfully.
I figured - perhaps it would be less taxing on the CNS, to add a drug with similar structural profile, in terms of its molecular make up - regardless of its ultimate pharmacological effect.
As three anti-depressants in combination is quite unusual - effectively attempting to scale back the "heavy'ness", of the approach, by having only slightly molecular disparity between two of them.
In this case - Atomoxetine and Fluoxetine - both being highly similar.
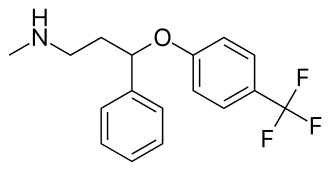
Fluoxetine
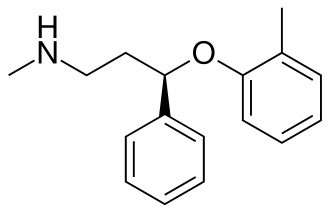
Atomoxetine - basically subbing the tri-fluoride ring for a carbon.
I'm on day 3 of this - and it's been the first drug I've taken in combo, that hasn't resulted in catastrophic sweating - so that's good.
I've upped the dose just now to 40 mg - as 20 mg falls below the standard 80% 5HTT occupancy.
Does this approach carry any merit?
Perhaps difficult to comment on as - it's certainly not been documented but - none the less.
Patterns of the universe, mathematical patterns, behavioral patterns etc - Fibonacci series is the one we're probably most familiar with.
Mathematicians have long since been attempting to determine patterns in the stock market.
Again - there's specifically no scientific data to document this in relation to pharmacology but - I've been using a combination of Atomoxetine and Mianserin to provide the most potent noradrenergic boost.
Subsequently - I have been attempting to introduce a serotonergic component via an SSRI/NRI type of drug - with limited success.
This is mainly due to their poor ability to combine well with the former two.
As Mianserin and mirtazapine basically have the same interaction profile - I thus attribute it to poor interaction potential with Atomoxetine - as SSRI/NRI's are well documented in combination with mirtazapine.
The only two large scale trials on Atomoxetine in combination has been with Sertraline, and fluoxetine.
I have attempted;
- Venlafaxine
- Escitalopram
- Sertraline
- Duloxetine
- Desvenlafaxine
- Brintellix
... as potential agents.
The closest I came was with Sertaline - and to a far lesser extent, Duloxetine.
I selected these on the basis of their CYP inhibition profiles (being low), as Atomoxetine is highly sensitive to CYP2D6 inhibition.
However - a very prevalent side effect - was excessive sweating.
This indicates to me, a highly taxed central nervous system.
Additionally - over the course of 24 hours - the best results were always had dosing, in between Atomoxetine and Mianserin - as I would normally dose these about 8:00, and 22:00.
So somewhere around 16:00 for the third agent.
This would suggest sufficient metabolic time for each agent.
My most recent attempt - has been with Fluoxetine.
I selected this as, looking for some kind of symmetry or pattern relative to the other two drugs - which I had previously sought in terms of the pharmacological profiles - unsuccessfully.
I figured - perhaps it would be less taxing on the CNS, to add a drug with similar structural profile, in terms of its molecular make up - regardless of its ultimate pharmacological effect.
As three anti-depressants in combination is quite unusual - effectively attempting to scale back the "heavy'ness", of the approach, by having only slightly molecular disparity between two of them.
In this case - Atomoxetine and Fluoxetine - both being highly similar.

Fluoxetine

Atomoxetine - basically subbing the tri-fluoride ring for a carbon.
I'm on day 3 of this - and it's been the first drug I've taken in combo, that hasn't resulted in catastrophic sweating - so that's good.
I've upped the dose just now to 40 mg - as 20 mg falls below the standard 80% 5HTT occupancy.
Does this approach carry any merit?
Perhaps difficult to comment on as - it's certainly not been documented but - none the less.
