(zonk)
Bluelighter
- Joined
- May 24, 2008
- Messages
- 687
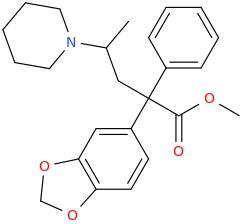
I was wondering if some simple reactions could be done to greatly increase it's bioavailability such as acetylating the hydroxy and changing the salt from HCl to a dehydroascorbate to help bypass pgp and carry it across the bbb? My supplier said this might be difficult because it's unstable in it's basic form and I'm not sure if dha is acidic enough in any amount to replace the HCl with basifying 1st. Anyway, would these modifications greatly increase bioavailability and would they be practical as I'm not familiar with it's stabilities?