Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
Just found this rather interesting depiction of bioisosteric requirements for DOR agonists, although with what selectivity I don't know.
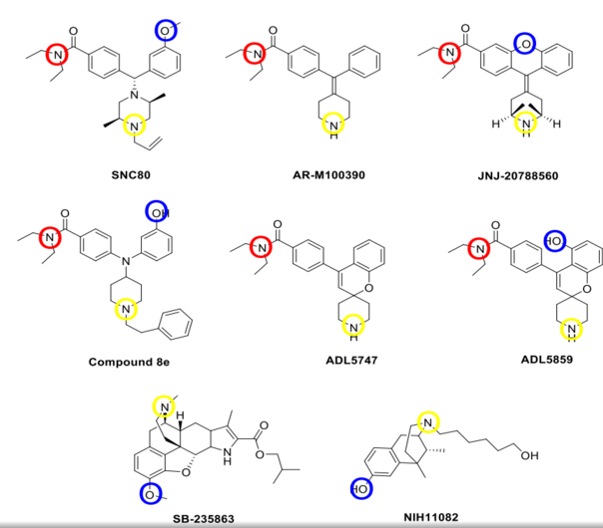
Look at SB-235863, now, I've not so much interest in the compound itself, but rather, whether the extra ring and it's sidechain are acting as a bioisostere for the diethyl amide seen in some of the benzhydryl-type compounds like SNC-80 to serve as something to stick in that hydrophobic pocket within the binding site, and how the common methoxy or sometimes OH group, always a hydrogen bond accptor might be substituted for a halogen, either in hydrogen or halogen bonding with the appropriate residue in the binding site to yield a DOR agonist.
Wondering because the bulky hydrophobic group is apparently not totally required, and would be absent from alpha-chloromorphide. Tried alpha-chloromorphide a while ago and it produced some odd effects I'd love to be able to assign at least likely suspect status to.
Effects were antinociceptive (I'm a chronic pain patient, it was tested whilst in withdrawal from MOR agonist opioids) but NOT like unto a MOR agonist opioid, it alleviated withdrawals partially, but to a relatively low extent. Effects perceived were primarily psychostimulant-like, but very different from either a DARI or TRI such as methyl/ethylphenidate and cocaine respectively, and also, totally unlike an amphetamine. Much more subtle and cerebral with almost no physical stimulation.
Nothing peripheral at all, no tachycardia, BP wasn't checked, was heading towards desomorphine via catalytic reduction, but that never got done at the time, as the intermediate proved far more intriguing, due to its odd nature. As the dose was pushed, the beginnings of what I am near certain would hav
Wondering because the bulky hydrophobic group is apparently not totally required, and would be absent from alpha-chloromorphide. Tried alpha-chloromorphide a while ago and it produced some odd effects I'd love to be able to assign at least likely suspect status to.
Effects were antinociceptive (I'm a chronic pain patient, it was tested whilst in withdrawal from MOR agonist opioids) but NOT like unto a MOR agonist opioid, it alleviated withdrawals partially, but to a relatively low extent. Effects perceived were primarily psychostimulant-like, but very different from either a DARI or TRI such as methyl/ethylphenidate and cocaine respectively, and also, totally unlike an amphetamine. Much more subtle and cerebral with almost no physical stimulation.
Nothing peripheral at all, no tachycardia, BP wasn't checked, was heading towards desomorphine via catalytic reduction, but that never got done at the time, as the intermediate proved far more intriguing, due to its odd nature. As the dose was pushed, the beginnings of what I am near certain would hav
Wondering because the bulky hydrophobic group is apparently not totally required, and would be absent from alpha-chloromorphide. Tried alpha-chloromorphide a while ago and it produced some odd effects I'd love to be able to assign at least likely suspect status to.
Effects were antinociceptive (I'm a chronic pain patient, it was tested whilst in withdrawal from MOR agonist opioids) but NOT like unto a MOR agonist opioid, it alleviated withdrawals partially, but to a relatively low extent. Effects perceived were primarily psychostimulant-like, but very different from either a DARI or TRI such as methyl/ethylphenidate and cocaine respectively, and also, totally unlike an amphetamine. Much more subtle and cerebral with almost no physical stimulation.
Nothing peripheral at all, no tachycardia, BP wasn't checked, was heading towards desomorphine via catalytic reduction, but that never got done at the time, as the intermediate proved far more intriguing, due to its odd nature. As the dose was pushed, the beginnings of what I am near certain would have been full blown convulsant properties seemed to emerge, with muscle jerks and clonus in the extremities, testing as such was stopped, I am seizure prone, although that day I was off my med (chlormethiazole) so I didn't want to push my luck and provoke things where such toxicity seemed likely. It felt a fair bit like the very first onset of a seizure, and I'm pretty sure it would have caused one had I increased the dose much above the range tested at all. So go figure, I'm all for science, not for poisoning myself however
So...two suspect modes of action. One being that it is a strychnine-sensitive GLyR antagonist, taking it's inspiration as such from things like thebaine That might fit with the profile of purely mental stimulation, although I've never tried strychnine or any other strychnine-sensitive GLyR antagonist other than the thebaine etc. content of pod tea, people used to use strychnine in low, low doses, up to maybe 1mg or so IIRC as a stimulant and 'tonic' as well as in attempts to rescue cases of respiratory depression (that was pretty much what they did back then, stuffed coffee up your arse and used strychnine, threw cold water over you etc. this is dating back to the 1700s when doctors were as likely to kill you as cure you and when heavy metals, strong acids, caustic minerals and toxic plants were pretty popular for all sorts of pretty shocking uses.
The other suspect, is a delta opioid agonist, At least some (implying lack of complete knowledge on my part, not that I know of DOR1 agonists specifically which do not) DOR1 agonists, fr.ex TAN-67 induce DA release in the nucleus accumbens, and we all know what THAT means don't we now?
Anyone here have experience directly with DOR-selective agonist ligands on an in-vivo basis? animal studies are all over the show, but you can't ask a rat what's going on in it's little murine mind.

Look at SB-235863, now, I've not so much interest in the compound itself, but rather, whether the extra ring and it's sidechain are acting as a bioisostere for the diethyl amide seen in some of the benzhydryl-type compounds like SNC-80 to serve as something to stick in that hydrophobic pocket within the binding site, and how the common methoxy or sometimes OH group, always a hydrogen bond accptor might be substituted for a halogen, either in hydrogen or halogen bonding with the appropriate residue in the binding site to yield a DOR agonist.
Wondering because the bulky hydrophobic group is apparently not totally required, and would be absent from alpha-chloromorphide. Tried alpha-chloromorphide a while ago and it produced some odd effects I'd love to be able to assign at least likely suspect status to.
Effects were antinociceptive (I'm a chronic pain patient, it was tested whilst in withdrawal from MOR agonist opioids) but NOT like unto a MOR agonist opioid, it alleviated withdrawals partially, but to a relatively low extent. Effects perceived were primarily psychostimulant-like, but very different from either a DARI or TRI such as methyl/ethylphenidate and cocaine respectively, and also, totally unlike an amphetamine. Much more subtle and cerebral with almost no physical stimulation.
Nothing peripheral at all, no tachycardia, BP wasn't checked, was heading towards desomorphine via catalytic reduction, but that never got done at the time, as the intermediate proved far more intriguing, due to its odd nature. As the dose was pushed, the beginnings of what I am near certain would hav
Wondering because the bulky hydrophobic group is apparently not totally required, and would be absent from alpha-chloromorphide. Tried alpha-chloromorphide a while ago and it produced some odd effects I'd love to be able to assign at least likely suspect status to.
Effects were antinociceptive (I'm a chronic pain patient, it was tested whilst in withdrawal from MOR agonist opioids) but NOT like unto a MOR agonist opioid, it alleviated withdrawals partially, but to a relatively low extent. Effects perceived were primarily psychostimulant-like, but very different from either a DARI or TRI such as methyl/ethylphenidate and cocaine respectively, and also, totally unlike an amphetamine. Much more subtle and cerebral with almost no physical stimulation.
Nothing peripheral at all, no tachycardia, BP wasn't checked, was heading towards desomorphine via catalytic reduction, but that never got done at the time, as the intermediate proved far more intriguing, due to its odd nature. As the dose was pushed, the beginnings of what I am near certain would hav
Wondering because the bulky hydrophobic group is apparently not totally required, and would be absent from alpha-chloromorphide. Tried alpha-chloromorphide a while ago and it produced some odd effects I'd love to be able to assign at least likely suspect status to.
Effects were antinociceptive (I'm a chronic pain patient, it was tested whilst in withdrawal from MOR agonist opioids) but NOT like unto a MOR agonist opioid, it alleviated withdrawals partially, but to a relatively low extent. Effects perceived were primarily psychostimulant-like, but very different from either a DARI or TRI such as methyl/ethylphenidate and cocaine respectively, and also, totally unlike an amphetamine. Much more subtle and cerebral with almost no physical stimulation.
Nothing peripheral at all, no tachycardia, BP wasn't checked, was heading towards desomorphine via catalytic reduction, but that never got done at the time, as the intermediate proved far more intriguing, due to its odd nature. As the dose was pushed, the beginnings of what I am near certain would have been full blown convulsant properties seemed to emerge, with muscle jerks and clonus in the extremities, testing as such was stopped, I am seizure prone, although that day I was off my med (chlormethiazole) so I didn't want to push my luck and provoke things where such toxicity seemed likely. It felt a fair bit like the very first onset of a seizure, and I'm pretty sure it would have caused one had I increased the dose much above the range tested at all. So go figure, I'm all for science, not for poisoning myself however

So...two suspect modes of action. One being that it is a strychnine-sensitive GLyR antagonist, taking it's inspiration as such from things like thebaine That might fit with the profile of purely mental stimulation, although I've never tried strychnine or any other strychnine-sensitive GLyR antagonist other than the thebaine etc. content of pod tea, people used to use strychnine in low, low doses, up to maybe 1mg or so IIRC as a stimulant and 'tonic' as well as in attempts to rescue cases of respiratory depression (that was pretty much what they did back then, stuffed coffee up your arse and used strychnine, threw cold water over you etc. this is dating back to the 1700s when doctors were as likely to kill you as cure you and when heavy metals, strong acids, caustic minerals and toxic plants were pretty popular for all sorts of pretty shocking uses.
The other suspect, is a delta opioid agonist, At least some (implying lack of complete knowledge on my part, not that I know of DOR1 agonists specifically which do not) DOR1 agonists, fr.ex TAN-67 induce DA release in the nucleus accumbens, and we all know what THAT means don't we now?

Anyone here have experience directly with DOR-selective agonist ligands on an in-vivo basis? animal studies are all over the show, but you can't ask a rat what's going on in it's little murine mind.
