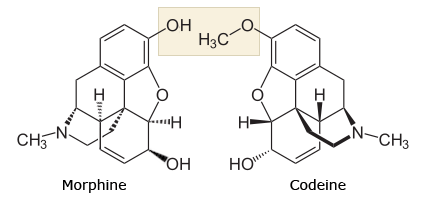
Hi all,
I've been wondering for a while now about the differences between morphone and codone drugs (hydrocodone vs. hydromorphone, oxycodone vs. oxymorphone for example)
What sets these two supposedly different drugs apart?
I see that often people claim to get a better high on "-morphone" drugs. Why is this?
When would "-morphone" drugs be used instead of "-codone" from a medical standpoint? (During surgery, certain cases, etc.. I have no intention of trying to get either of these types of drugs, simply curious.)
Thanks for the help.
I've been wondering for a while now about the differences between morphone and codone drugs (hydrocodone vs. hydromorphone, oxycodone vs. oxymorphone for example)
What sets these two supposedly different drugs apart?
I see that often people claim to get a better high on "-morphone" drugs. Why is this?
When would "-morphone" drugs be used instead of "-codone" from a medical standpoint? (During surgery, certain cases, etc.. I have no intention of trying to get either of these types of drugs, simply curious.)
Thanks for the help.