Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
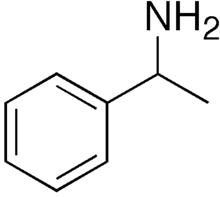
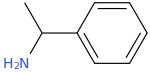
Without going any further into sourcing territory than simply 'as typically sold as a 'supplement', is this typically a racemate, or would taking it to the lab to perform a chiral resolution be necessary? as I've just been reading some interesting material on transamination of certain aryl alkyl ketones using Arthrobacter strains using 1-phenethylamine as an amino-donor, such as phenylacetones to amphetamines in an enantioselective manner, and substituted acetophenones to the corresponding phenethylamines. This is enantioselective for the R-isomer, whilst Brevibacterium linens (from limburger cheese) is capable of a similar feat, but selective for the S-isomer of the amino-donor, which at least in the former case, the transaminase enzyme can be induced by exposure to low levels of sec-butylamine.
Just thought it would be a really interesting experiment, to attempt an enzymatic enantioselective biosynthesis of D-amphetamine from unsubstituted P2P, since P2P routes to this desirable compound typically result in a racemic product (question-since the corresponding ketoxime is achiral, what is the result, stereochemistry-wise of boveault-blanc reduction of P2P ketoximes?)
Just thought it would be a really interesting experiment, to attempt an enzymatic enantioselective biosynthesis of D-amphetamine from unsubstituted P2P, since P2P routes to this desirable compound typically result in a racemic product (question-since the corresponding ketoxime is achiral, what is the result, stereochemistry-wise of boveault-blanc reduction of P2P ketoximes?)