Volsam
Bluelighter
- Joined
- Nov 19, 2016
- Messages
- 909
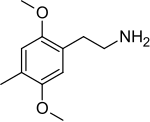
2C-D
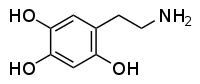 Oxidopamine
Oxidopamine
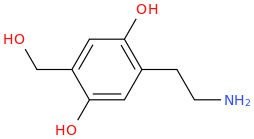
I was reading about metabolic pathways of 2C-D: o-demethylation in positions 2 and 5, hydroxylation in the alkyl radical in the 4th position (these two steps pretty much make 2C-D into Oxidopamine), N-acetylation, beta-hydroxylation and deamination with subsequent oxidation to carboxylic acid or reduction to alcohol.
Does that mean that at some point of it's metabolic process 2C-D may become a neurotoxic Oxidopamine (2,4,5-trihydroxyphenethylamine) that destroys dopaminergic and noradrenergic neurons in the brain?


I was reading about metabolic pathways of 2C-D: o-demethylation in positions 2 and 5, hydroxylation in the alkyl radical in the 4th position (these two steps pretty much make 2C-D into Oxidopamine), N-acetylation, beta-hydroxylation and deamination with subsequent oxidation to carboxylic acid or reduction to alcohol.
Does that mean that at some point of it's metabolic process 2C-D may become a neurotoxic Oxidopamine (2,4,5-trihydroxyphenethylamine) that destroys dopaminergic and noradrenergic neurons in the brain?