https://imgur.com/MpSH05v
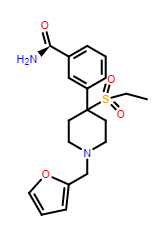
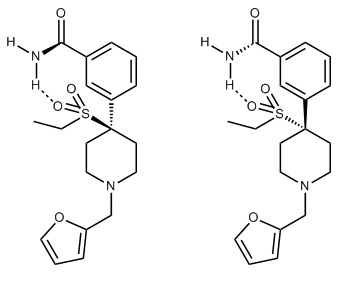
So, we have a chiral carboxamide as a bioisostere of a phenolic group, an ethylsulfonyl group as bioisostere of ethyl ketone and shock - an N-2-furanylbenzyl as bioisostere of the common or garden N-ethylaryl. I have an unknown. Substitution of the piperidine ring is supposed to be verboten for ketobemidones BUT picenadol (LY-97435) suggests the reason why such substitution failed was simply because like many phenolic opioids, they can be agonists and/or antagonists. I dare not believe that swapping the methyl for an allyl would engender a serious increase in potency BUT it does raise the issue of what has been left untested. When the side-chain bares no oxygen, the chain-length is important. A methyl is an antagonist, an ethyl is a partial agonist (see meptazinol) while (S)-picenadol is an agonist.
It has nothing special to make it a clandestine target but as a series including the 2,5-dimethyl as well as various 3-n-alkyls and N-benzylaryl groups (thiophene, thiazole, oxazole & isoazole for example) WOULD be of tremendous value as a training set. MANY 5 & 6 membered aromatic rings have been placed onto the anilidopiperidine scaffold and many times more on the dual mu/delta ligands that have gone nowhere but the example I give is one of around x20 morphine based on the (English) patents.
Going even simpler, dimethylaminopivalophenone overlays 3,3-dimethylpethidine BUT the position of the O is the same as one of the Os in allylprodine so what activity would people predict for dimethylamino-3-allyl-3-methyl-heptephenone (suppose it's about as accurate as the original!). If the allyl works I predict that replacing the N-methyl for an N-allyl in U-47700 will have pronounced effects.
So, we have a chiral carboxamide as a bioisostere of a phenolic group, an ethylsulfonyl group as bioisostere of ethyl ketone and shock - an N-2-furanylbenzyl as bioisostere of the common or garden N-ethylaryl. I have an unknown. Substitution of the piperidine ring is supposed to be verboten for ketobemidones BUT picenadol (LY-97435) suggests the reason why such substitution failed was simply because like many phenolic opioids, they can be agonists and/or antagonists. I dare not believe that swapping the methyl for an allyl would engender a serious increase in potency BUT it does raise the issue of what has been left untested. When the side-chain bares no oxygen, the chain-length is important. A methyl is an antagonist, an ethyl is a partial agonist (see meptazinol) while (S)-picenadol is an agonist.
It has nothing special to make it a clandestine target but as a series including the 2,5-dimethyl as well as various 3-n-alkyls and N-benzylaryl groups (thiophene, thiazole, oxazole & isoazole for example) WOULD be of tremendous value as a training set. MANY 5 & 6 membered aromatic rings have been placed onto the anilidopiperidine scaffold and many times more on the dual mu/delta ligands that have gone nowhere but the example I give is one of around x20 morphine based on the (English) patents.
Going even simpler, dimethylaminopivalophenone overlays 3,3-dimethylpethidine BUT the position of the O is the same as one of the Os in allylprodine so what activity would people predict for dimethylamino-3-allyl-3-methyl-heptephenone (suppose it's about as accurate as the original!). If the allyl works I predict that replacing the N-methyl for an N-allyl in U-47700 will have pronounced effects.