Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
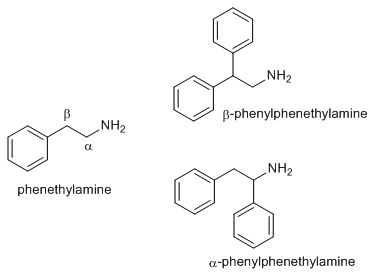
What is known of beta-phenylamphetamine? Also, beta-phenyl-N-methyl and beta-Ph-N-ethylamphetamine?
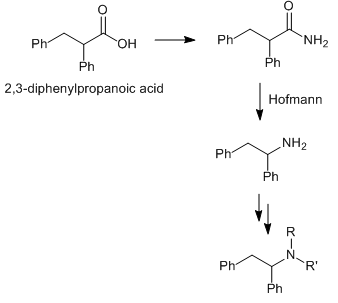
Most interested in the primary amine however, since from diphenylacetic acid looks like a two-step reaction, forming a nitrile using diphosphorus tetraiodide in anhydrous carbon disulfide at RT for a couple of hours, then reduction of the nitrile via in-situ formation of STAB, with the nitrile in GAA solution and addition of NaBH4.
I've heard before that B-phenylmethamphetamine is both active, euphoric and long lasting, never heard of
the primary amine being used before, nor beta-phenyl-N-ethylamphetamine.
Most interested in the primary amine however, since from diphenylacetic acid looks like a two-step reaction, forming a nitrile using diphosphorus tetraiodide in anhydrous carbon disulfide at RT for a couple of hours, then reduction of the nitrile via in-situ formation of STAB, with the nitrile in GAA solution and addition of NaBH4.
I've heard before that B-phenylmethamphetamine is both active, euphoric and long lasting, never heard of
the primary amine being used before, nor beta-phenyl-N-ethylamphetamine.