MrBadExample
Greenlighter
- Joined
- Jan 9, 2018
- Messages
- 8
Hi,
This will be a long-ish post, as I shall try to be as clear and detailed as possible.
I have been interested, for a while now, in purifying poppy tea by some means. The purpose of which is to reach a sufficient purity to allow the alkaloids to be vaporized.
I have done countless experiments with chemical acid-base extractions (with no success, for various reasons).
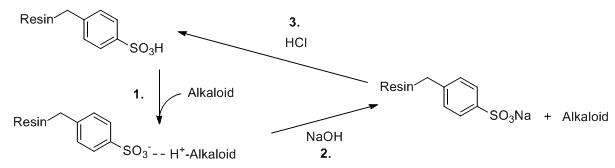
Upon further research, I found out about Polish Heroin/Kompot, and the interesting (and seemingly fool-proof) method of using a polystyrene sulphonic acid cation exchange resin to extract the alkaloids from poppy tea.
Enthused by this possibility, I acquired some of the resin and proceeded to try it out.
Before I go on to explain the problems I have encountered, let me first describe some of the unusual observations I've made so far...
As stated above, all attempts that I have made to extract the alkaloids by chemical means have failed for reasons that are no longer particularly relevant, however there is something, that I cannot as yet explain, that happened during every attempt of acid-base extraction -
Upon addition of a base to the poppy tea, ammonia is evolved and the tea is thereafter seemingly inactive and no longer bitter (although I wasn't too enthusiastic about drinking large quantities of alkaline tea to test for narcotic activity)
For the base I have used sodium carbonate, sodium hydroxide, and calcium hydroxide, all with the same result - As soon as the base is added, the smell of ammonia is easily observed and it seems to continue to be evolved over a period of an hour or so (although this may be due to the dissolved ammonia evaporating out of solution).
The smell isn't strong, as I can easily hold it up to my nose without choking, but it is definitely ammonia.
Does anyone have any ideas about what could be happening here?
As far as I know, morphine and related alkaloids, in the presence of a strong base, form phenoxides of the base. In this case sodium morphenate would form:
R-OH + NaOH --> R-ONa + H2O
Any fats and oils present in the poppy tea will saponify, and other stuff like lactic and meconic acids will form salts with the base.
None of these reactions explain the evolution of ammonia - Could it be that the alkaloids are decomposing somehow?
I have attempted to make pod putty by evaporating poppy tea. This has been a partial success, as the end result is 6x purer than the poppy straw by weight, however it seems to take on a different nature as if some of the alkaloids have been denatured by the heating.
This seems unlikely as it was heated to only ~60C (albeit over a period of around a day)
Anyway, back to ion-exchange chemisorption:
I did a bit of reading about how ion-exchange resins work, and I learned that, in this case, it works because the SO2OH groups have a higher affinity for heavier, higher valency ions, such as morphine, than for lighter monovalent ions such as sodium.
I was initially confused by how the reaction is reversible, until I learned that the reaction is reversed by exposing the resin with a very strong solution containing ions for which the resin has a lower affinity, this causes the alkaloids to be displaced into the solution.
So I made poppy tea with multiple extractions by the usual method, using 10g of poppy straw.
The tea tasted very bitter.
To it was added 20g of ion-exchange resin, which was mixed thoroughly over a period of hours using a motor, after which it was filtered from the tea.
At this point, the tea had turned a lighter colour and tasted completely free of bitterness, it had a sweet, almost saline taste.
The tea was poured away and the resin was rinsed with a small volume of cold water.
Now, I wasn't sure what to do next. I had read a dubious Kompot tek (http://www.chemart.republika.pl/atomy/jak_zrobic_heroine.html) that suggested using ammonia to extract the alkaloids from the resin, however I elected instead to use hydrochloric acid for the following reasons:
My previous experience showed that bases seem to have weird effects on poppy tea, so perhaps they denature the alkaloids.
The opium alkaloids are mostly insoluble in bases, whereas they are freely soluble in acid, so there would be no chance of accidentally filtering out the precipitated alkaloids when straining the resin from the solution if I used acid.
Having decided so use HCl, I added the resin to 10mL of 35% HCl and allowed it to sit for a while (in later attempts I tried mixing it more thoroughly, and heating it, neither of which made any obvious difference).
The solution turned light-yellow, whereupon I filtered out the resin, retained it, and evaporated off the acidic solution.
Unfortunately, no alkaloids were obtained, only large quantities of sodium chloride.
I ran alcohol over the sodium chloride just in case traces of alkaloids were mixed in with the salt, to no avail.
At this point I realised my mistake in having used the sodium-treated ion exchange resin, instead of first treating it with an acid to replace the R-SO2-ONa groups with R-SO2-OH...
Nonetheless, I repeated the process several times in the hope that, once the sodium had been displaced entirely from the resin, the alkaloids would begin to be displaced by the strong HCl.
This has had no success so far...
One last thing, the Kompot tek that I linked has something that makes absolutely no sense to me, it states that the resin should be pre-treated with a solution of NaCl AND either acetic or hydrochloric acid.
Why would this be? If the objective is to convert all of the sulphonate groups to sulphonic acid groups, displacing any sodium, why use NaCl? And if, conversely, the objective is to turn the sulphonic acid groups into sodium-R-sulphonate groups, why use any acid?
I would greatly appreciate any help you could offer.
Do you think my mistake was to fail to remove the sodium from the resin in the first place?
Thanks a lot for your time.
Love from Mr Bad Example
This will be a long-ish post, as I shall try to be as clear and detailed as possible.
I have been interested, for a while now, in purifying poppy tea by some means. The purpose of which is to reach a sufficient purity to allow the alkaloids to be vaporized.
I have done countless experiments with chemical acid-base extractions (with no success, for various reasons).
Upon further research, I found out about Polish Heroin/Kompot, and the interesting (and seemingly fool-proof) method of using a polystyrene sulphonic acid cation exchange resin to extract the alkaloids from poppy tea.
Enthused by this possibility, I acquired some of the resin and proceeded to try it out.
Before I go on to explain the problems I have encountered, let me first describe some of the unusual observations I've made so far...
As stated above, all attempts that I have made to extract the alkaloids by chemical means have failed for reasons that are no longer particularly relevant, however there is something, that I cannot as yet explain, that happened during every attempt of acid-base extraction -
Upon addition of a base to the poppy tea, ammonia is evolved and the tea is thereafter seemingly inactive and no longer bitter (although I wasn't too enthusiastic about drinking large quantities of alkaline tea to test for narcotic activity)
For the base I have used sodium carbonate, sodium hydroxide, and calcium hydroxide, all with the same result - As soon as the base is added, the smell of ammonia is easily observed and it seems to continue to be evolved over a period of an hour or so (although this may be due to the dissolved ammonia evaporating out of solution).
The smell isn't strong, as I can easily hold it up to my nose without choking, but it is definitely ammonia.
Does anyone have any ideas about what could be happening here?
As far as I know, morphine and related alkaloids, in the presence of a strong base, form phenoxides of the base. In this case sodium morphenate would form:
R-OH + NaOH --> R-ONa + H2O
Any fats and oils present in the poppy tea will saponify, and other stuff like lactic and meconic acids will form salts with the base.
None of these reactions explain the evolution of ammonia - Could it be that the alkaloids are decomposing somehow?
I have attempted to make pod putty by evaporating poppy tea. This has been a partial success, as the end result is 6x purer than the poppy straw by weight, however it seems to take on a different nature as if some of the alkaloids have been denatured by the heating.
This seems unlikely as it was heated to only ~60C (albeit over a period of around a day)
Anyway, back to ion-exchange chemisorption:
I did a bit of reading about how ion-exchange resins work, and I learned that, in this case, it works because the SO2OH groups have a higher affinity for heavier, higher valency ions, such as morphine, than for lighter monovalent ions such as sodium.
I was initially confused by how the reaction is reversible, until I learned that the reaction is reversed by exposing the resin with a very strong solution containing ions for which the resin has a lower affinity, this causes the alkaloids to be displaced into the solution.
So I made poppy tea with multiple extractions by the usual method, using 10g of poppy straw.
The tea tasted very bitter.
To it was added 20g of ion-exchange resin, which was mixed thoroughly over a period of hours using a motor, after which it was filtered from the tea.
At this point, the tea had turned a lighter colour and tasted completely free of bitterness, it had a sweet, almost saline taste.
The tea was poured away and the resin was rinsed with a small volume of cold water.
Now, I wasn't sure what to do next. I had read a dubious Kompot tek (http://www.chemart.republika.pl/atomy/jak_zrobic_heroine.html) that suggested using ammonia to extract the alkaloids from the resin, however I elected instead to use hydrochloric acid for the following reasons:
My previous experience showed that bases seem to have weird effects on poppy tea, so perhaps they denature the alkaloids.
The opium alkaloids are mostly insoluble in bases, whereas they are freely soluble in acid, so there would be no chance of accidentally filtering out the precipitated alkaloids when straining the resin from the solution if I used acid.
Having decided so use HCl, I added the resin to 10mL of 35% HCl and allowed it to sit for a while (in later attempts I tried mixing it more thoroughly, and heating it, neither of which made any obvious difference).
The solution turned light-yellow, whereupon I filtered out the resin, retained it, and evaporated off the acidic solution.
Unfortunately, no alkaloids were obtained, only large quantities of sodium chloride.
I ran alcohol over the sodium chloride just in case traces of alkaloids were mixed in with the salt, to no avail.
At this point I realised my mistake in having used the sodium-treated ion exchange resin, instead of first treating it with an acid to replace the R-SO2-ONa groups with R-SO2-OH...
Nonetheless, I repeated the process several times in the hope that, once the sodium had been displaced entirely from the resin, the alkaloids would begin to be displaced by the strong HCl.
This has had no success so far...
One last thing, the Kompot tek that I linked has something that makes absolutely no sense to me, it states that the resin should be pre-treated with a solution of NaCl AND either acetic or hydrochloric acid.
Why would this be? If the objective is to convert all of the sulphonate groups to sulphonic acid groups, displacing any sodium, why use NaCl? And if, conversely, the objective is to turn the sulphonic acid groups into sodium-R-sulphonate groups, why use any acid?
I would greatly appreciate any help you could offer.
Do you think my mistake was to fail to remove the sodium from the resin in the first place?
Thanks a lot for your time.
Love from Mr Bad Example