Volsam
Bluelighter
- Joined
- Nov 19, 2016
- Messages
- 909
I like researching drugs molecules, read about binding affinities, pharmacology and all that suff, but I have no chemical study background, nor any medical education, so I do it simply for fun.
I have recently gotten a rather atypical antidepressant from Russia, Afobazole (Fabomotizole) and was reading about it's action - turns out that it's antidepressive qualities may come from agonism on sigma-1 receptor. So I started reading more on this and was a little surprised to find DMT, PCP, Cocaine, DXM, Methamphetamine and all other addictive and nicely active stuff as agonists (or antagonists) on this receptor. 8)
8)
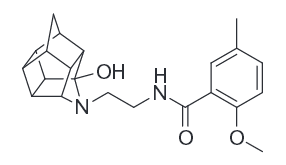
I have also found a peculiar similarities in molecular structures of most of the ligands - they all contain phenyl ring and a nitrogen having 3 methyls attached to it in a triangle shape (like DMT) or 2 methyls and a hydrogen (like Ketamine or Methamphetamine), with exception of hormones and hormonal steroids, like Progesterone, DHEA and Pregnenolone (they probably exert their action through Pregnane X receptor?..).
Made a little picture with some of the molecules that bind to sigma receptor:

I may be stating something very obvious here but those structures similarities appeared rather interesting and not mentioned anywhere.
I'm wondering what does it really take (scientifically proven) for a molecule to bind to sigma receptors and why is there barely any research in this area?
From Wiki: "The function of these receptors is poorly understood.[4] Activation of σ–receptors by an agonist ligand may induce hallucinogenic effects and also may be responsible for the paradoxical convulsions sometimes seen in opiate overdose. Physiologic effects when the σ–receptor is activated include hypertonia, tachycardia, tachypnea, antitussive effects, and mydriasis. Some σ–receptor agonists—such as cocaine, a weak σ–agonist—exert convulsant effects in animals. Behavioral reactions to σ–agonists are rather heterogeneous: some individuals find σ–receptor agonists euphoric with significant anti-depressive effects. Other individuals, however, experience dysphoria and often report feelings of malaise or anxiety. Recently selective σ–receptor agonists were shown to produce antidepressant-like effects in mice.[8]"
Seems like a more thorough research might yield new discoveries on how our physical brain is connected to consciousness, mental deficiencies and a sense of happiness and euphoria as well as perhaps an understanding how cancer has anything to do with our lifestyle and chemistry.
What do you guys think of it?
I have recently gotten a rather atypical antidepressant from Russia, Afobazole (Fabomotizole) and was reading about it's action - turns out that it's antidepressive qualities may come from agonism on sigma-1 receptor. So I started reading more on this and was a little surprised to find DMT, PCP, Cocaine, DXM, Methamphetamine and all other addictive and nicely active stuff as agonists (or antagonists) on this receptor.
 8)
8)I have also found a peculiar similarities in molecular structures of most of the ligands - they all contain phenyl ring and a nitrogen having 3 methyls attached to it in a triangle shape (like DMT) or 2 methyls and a hydrogen (like Ketamine or Methamphetamine), with exception of hormones and hormonal steroids, like Progesterone, DHEA and Pregnenolone (they probably exert their action through Pregnane X receptor?..).
Made a little picture with some of the molecules that bind to sigma receptor:

I may be stating something very obvious here but those structures similarities appeared rather interesting and not mentioned anywhere.
I'm wondering what does it really take (scientifically proven) for a molecule to bind to sigma receptors and why is there barely any research in this area?
From Wiki: "The function of these receptors is poorly understood.[4] Activation of σ–receptors by an agonist ligand may induce hallucinogenic effects and also may be responsible for the paradoxical convulsions sometimes seen in opiate overdose. Physiologic effects when the σ–receptor is activated include hypertonia, tachycardia, tachypnea, antitussive effects, and mydriasis. Some σ–receptor agonists—such as cocaine, a weak σ–agonist—exert convulsant effects in animals. Behavioral reactions to σ–agonists are rather heterogeneous: some individuals find σ–receptor agonists euphoric with significant anti-depressive effects. Other individuals, however, experience dysphoria and often report feelings of malaise or anxiety. Recently selective σ–receptor agonists were shown to produce antidepressant-like effects in mice.[8]"
Seems like a more thorough research might yield new discoveries on how our physical brain is connected to consciousness, mental deficiencies and a sense of happiness and euphoria as well as perhaps an understanding how cancer has anything to do with our lifestyle and chemistry.
What do you guys think of it?