aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
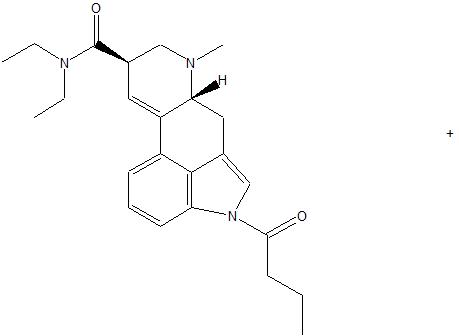
6-BU-LAD is LSD with a butyl group substituted on the basic nitrogen, not the indole nitrogen. Just for everyone's information, 6-butyl-LSD has been synthesised, and it was about 10 times less potent than LSD in rat drug discrimination.
"Lysergamides Revisited": https://archives.drugabuse.gov/pdf/monographs/146.pdf
Generally 1-substitution will attenuate activity but indolic amides get hydrolysed much easier than normal amides.
"Lysergamides Revisited": https://archives.drugabuse.gov/pdf/monographs/146.pdf
Generally 1-substitution will attenuate activity but indolic amides get hydrolysed much easier than normal amides.