Nagelfar
Bluelight Crew
Anybody know of a website that elucidates some of the potential transition metal pi-stacking complexes for benzenes?
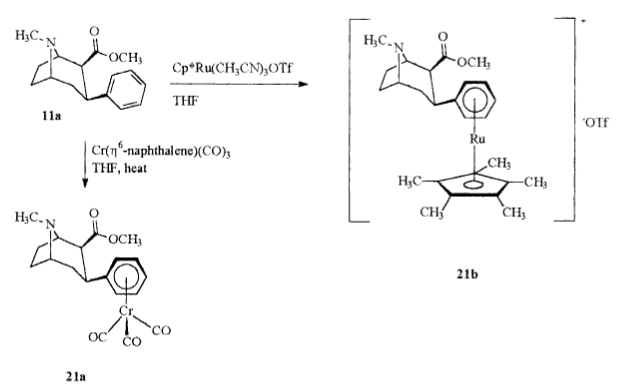
Ever since reading how the sandwich-stacked chromium-tricarbonyl (eta6-coordination moiety substitution) of troparil doubled its binding strength, I have been wondering if it really isn't just an issue of asymmetrical volume of the otherwise planar arene/aryl. It'd be interesting to see what T-shaped or parrallel-displaced pi-stacked C3 modifications do to considerations of binding @ the DAT.
My other question is whether the pentamethyl interferes with the H5 of the C6H5 remaining places on the phenyl, whether the tricarbonyl stick out the same way to likewise interfere, and if so, if there aren't at least 2 free spaces then on the phenyl with the tri-substituion-stacked versus the penta-substitution-stacked.
Any good resources in this vein at all would be greatly appreciated.
Ever since reading how the sandwich-stacked chromium-tricarbonyl (eta6-coordination moiety substitution) of troparil doubled its binding strength, I have been wondering if it really isn't just an issue of asymmetrical volume of the otherwise planar arene/aryl. It'd be interesting to see what T-shaped or parrallel-displaced pi-stacked C3 modifications do to considerations of binding @ the DAT.
My other question is whether the pentamethyl interferes with the H5 of the C6H5 remaining places on the phenyl, whether the tricarbonyl stick out the same way to likewise interfere, and if so, if there aren't at least 2 free spaces then on the phenyl with the tri-substituion-stacked versus the penta-substitution-stacked.
Any good resources in this vein at all would be greatly appreciated.