Isochromium
Bluelighter
- Joined
- Mar 25, 2013
- Messages
- 59
GOD = Generator Operator Destroyer
It will not be known where the limit of power is, until that limit is exceeded.
This thread is the first of two; the second thread's subject will be the design of toxic cannabinoids containing heavy metals, radioactives and halogens as integral components of their structures. It was inspired by the Fluorinated cannabinoids and their notorious side-effects. The therapeutic activity of toxic cannabinoids will be via either stacking or the formation of covalent bonds with membrane CB receptors and murder of the cell by radioactivity and/or toxicity. Considering the potency of stars such as 5F-AMB, it seems that molecule in particular could be used to deliver in seconds to highly targeted sites - the CB receptors or perhaps entire cells - a dose of lethality. Thus, the cannabinoids are not just intoxicants or medicines but also weapons. Chemical weapons.
It seems that much stronger cannabinoids might be designed based on observations of known cannabinoids.
From a certain Russian report it is known that there exists such a very loving molecule:
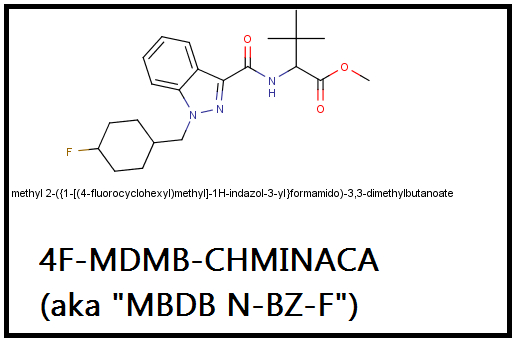
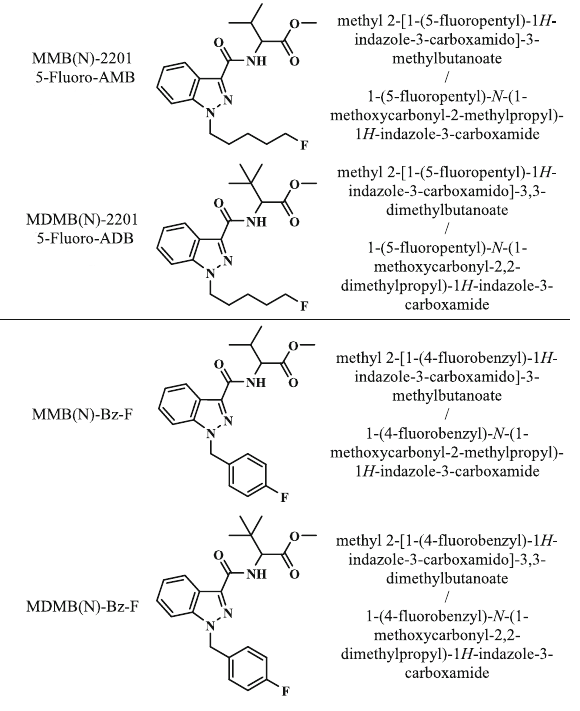
4F-MDMB-CHMINACA (MDMB N-BZ-F)
Methyl 2-({1-[(4-fluorobenzyl)methyl]-1H-indazol-3-yl}formamido)-3,3-dimethylbutanoate
"Russia’s Health Ministry has recently reported on 25 synthetic cannabinoid-related deaths, along with more than 700 hospitalizations. The substance in question is simply described as MDMB N-Bz F. Average age of the hospitalizations is estimated at 24. According to this article, the substance has sent users “into vegetative state, provoking dementia and even respiratory arrest.” Other reports of this substance include a male who stabbed himself several times after smoking the synthetic cannabinoid product. Russia’s Federal Drug Control Service (FDCS) has taken steps to control the substance (as well as any other substance “for a year the moment they are discovered”)."
Really this molecule is in the AMB-series due to the terminal methoxy, and its indazole core means I think it ought to be called 4F-MDMB-AMB.
It might just be the closest to a covalent bond that can be had - that 'vegetative state' sounds persistent.
Nevertheless, I am still convinced potency and peak power improvements are waiting behind unexplored doors.
It seems there are some lessons to be learned from existing potency winners:
It just seemed to me that the grave cross reminded me of another notorious psychoactive hallucinogen and toxic antimalarial: Mefloquine.
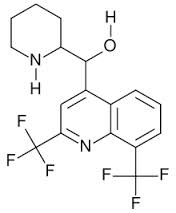
Mefloquine[(R*,S*)-2,8-bis(trifluoromethyl)quinolin-4-yl]-(2-piperidyl)methanol
And it is so nice to have so many files on so many drugs because I remembered all the Mefloquine stories. It's my favorite horror drug. It's impossible not to notice how those two trifluoromethyl groups give Mefloquine its kick. Their electronegativity is unbeatable which is why they are there. They were put there because just as Fluorine does not belong in any biosystem due to its tendency to too-tight bonds with carbon and excessive electronegativity, this is an antibiotic. Its job is to kill cells. Malarial cells and also brain cells. I can just begin to guess all the biomachinery that those two trifluoromethyls jam up. It's an ugly story. As bad as the Fluoroquinolones or worse.
Best yet, I can borrow something from Mefloquine and from the US Walter Reed Army Hospital's design work.
My goal is to design a horror that can match Mefloquine and make it for cannabinoid receptors.
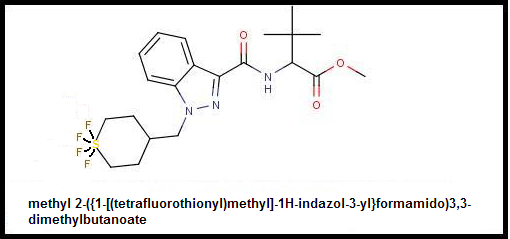
There are two most obvious potentials and the first is substitution of the 4F-MDMB-CHMINACA's dimethylbutanoate for a nice trifluoromethyl which in addition to being insanely electronegative which will make effects unpredictable:
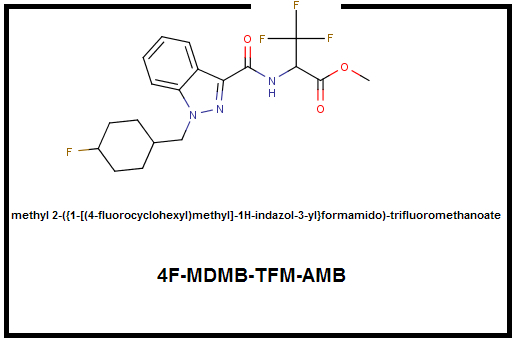
4F-MDMB-TFM-AMB
Methyl 2-({1-[(4-fluorocyclohexyl)methyl]-1H-indazol-3-yl}formamido)-trifluoromethanoate
My real hope and my real life and death bet however is that well-known para-fluoro on the pentyl/cyclohexyl/phenyl. It is well-known that the highly electronegative fluoro at the end of the pentyl chain, or the para-tip of the ring, enormously increases the molecule's activation potency at CB1 and generally increases overall CB potency by a large quantity.
Besides that electronegative fluoro at the tip it seems that a fairly bulky hydrocarbon chain or that hydrocarbon cyclohexyl work well. Phenyl works too but I can find only AB-FUBINACA that uses it. There are not enough proven molecules using the phenyl group to offer a definitive conclusion.
Thus comes the first means to increase both electronegativity and bulk by modifying the olde Russian 4F-MDMB-CHMINACA thusly:
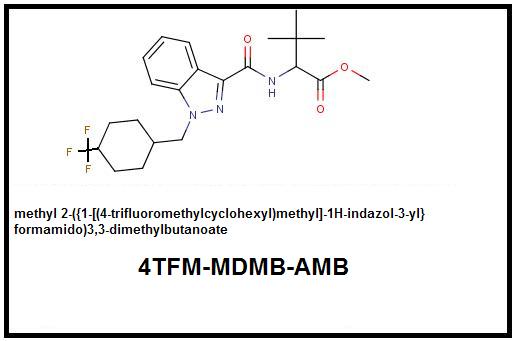
It seems there is one other candidate for this modification and it is 5F-AMB which even now exists as a terror molecule of high quality:
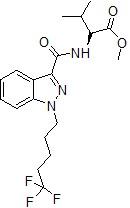
5TFM-AMB
I am very sure that 5TFM-AMB is a cannabinoid and I am also convinced that it is a chemical weapon.
I feel absolute terror when I look at 5TFM-AMB. It's because 5F-AMB was not the end of the road.
The end of this road is the place of GOD: Generator, Operator, Destroyer.
A custom synthesis should be easy given 5TFM-AMB'sclose relation with 5F-AMB.
The final step is militarization which given likely potency ought to be feasible.
It will not be known where the limit of power is, until that limit is exceeded.
This thread is the first of two; the second thread's subject will be the design of toxic cannabinoids containing heavy metals, radioactives and halogens as integral components of their structures. It was inspired by the Fluorinated cannabinoids and their notorious side-effects. The therapeutic activity of toxic cannabinoids will be via either stacking or the formation of covalent bonds with membrane CB receptors and murder of the cell by radioactivity and/or toxicity. Considering the potency of stars such as 5F-AMB, it seems that molecule in particular could be used to deliver in seconds to highly targeted sites - the CB receptors or perhaps entire cells - a dose of lethality. Thus, the cannabinoids are not just intoxicants or medicines but also weapons. Chemical weapons.
It seems that much stronger cannabinoids might be designed based on observations of known cannabinoids.
From a certain Russian report it is known that there exists such a very loving molecule:

4F-MDMB-CHMINACA (MDMB N-BZ-F)
Methyl 2-({1-[(4-fluorobenzyl)methyl]-1H-indazol-3-yl}formamido)-3,3-dimethylbutanoate
"Russia’s Health Ministry has recently reported on 25 synthetic cannabinoid-related deaths, along with more than 700 hospitalizations. The substance in question is simply described as MDMB N-Bz F. Average age of the hospitalizations is estimated at 24. According to this article, the substance has sent users “into vegetative state, provoking dementia and even respiratory arrest.” Other reports of this substance include a male who stabbed himself several times after smoking the synthetic cannabinoid product. Russia’s Federal Drug Control Service (FDCS) has taken steps to control the substance (as well as any other substance “for a year the moment they are discovered”)."
Really this molecule is in the AMB-series due to the terminal methoxy, and its indazole core means I think it ought to be called 4F-MDMB-AMB.
It might just be the closest to a covalent bond that can be had - that 'vegetative state' sounds persistent.
Nevertheless, I am still convinced potency and peak power improvements are waiting behind unexplored doors.
It seems there are some lessons to be learned from existing potency winners:
1. Fluoropentyl or Parafluoro-cycle on the Indazole. Jury's out on which one might be 'strongest' and it might depend on the rest of the molecule.
2. That amazing terminal methoxy which makes the AMB series so much more potent than CHMINACAs.
3. The grave cross or cross of death. It's called the dimethylbutanoate aka. tert-butyl. The grave cross replaces the older, less-potent isopropyl group. The lesson here is bulkier groups = stronger effects.
2. That amazing terminal methoxy which makes the AMB series so much more potent than CHMINACAs.
3. The grave cross or cross of death. It's called the dimethylbutanoate aka. tert-butyl. The grave cross replaces the older, less-potent isopropyl group. The lesson here is bulkier groups = stronger effects.
It just seemed to me that the grave cross reminded me of another notorious psychoactive hallucinogen and toxic antimalarial: Mefloquine.

Mefloquine[(R*,S*)-2,8-bis(trifluoromethyl)quinolin-4-yl]-(2-piperidyl)methanol
And it is so nice to have so many files on so many drugs because I remembered all the Mefloquine stories. It's my favorite horror drug. It's impossible not to notice how those two trifluoromethyl groups give Mefloquine its kick. Their electronegativity is unbeatable which is why they are there. They were put there because just as Fluorine does not belong in any biosystem due to its tendency to too-tight bonds with carbon and excessive electronegativity, this is an antibiotic. Its job is to kill cells. Malarial cells and also brain cells. I can just begin to guess all the biomachinery that those two trifluoromethyls jam up. It's an ugly story. As bad as the Fluoroquinolones or worse.
Best yet, I can borrow something from Mefloquine and from the US Walter Reed Army Hospital's design work.
My goal is to design a horror that can match Mefloquine and make it for cannabinoid receptors.
There are two most obvious potentials and the first is substitution of the 4F-MDMB-CHMINACA's dimethylbutanoate for a nice trifluoromethyl which in addition to being insanely electronegative which will make effects unpredictable:

4F-MDMB-TFM-AMB
Methyl 2-({1-[(4-fluorocyclohexyl)methyl]-1H-indazol-3-yl}formamido)-trifluoromethanoate
My real hope and my real life and death bet however is that well-known para-fluoro on the pentyl/cyclohexyl/phenyl. It is well-known that the highly electronegative fluoro at the end of the pentyl chain, or the para-tip of the ring, enormously increases the molecule's activation potency at CB1 and generally increases overall CB potency by a large quantity.
Besides that electronegative fluoro at the tip it seems that a fairly bulky hydrocarbon chain or that hydrocarbon cyclohexyl work well. Phenyl works too but I can find only AB-FUBINACA that uses it. There are not enough proven molecules using the phenyl group to offer a definitive conclusion.
Thus comes the first means to increase both electronegativity and bulk by modifying the olde Russian 4F-MDMB-CHMINACA thusly:

It seems there is one other candidate for this modification and it is 5F-AMB which even now exists as a terror molecule of high quality:

5TFM-AMB
I am very sure that 5TFM-AMB is a cannabinoid and I am also convinced that it is a chemical weapon.
I feel absolute terror when I look at 5TFM-AMB. It's because 5F-AMB was not the end of the road.
The end of this road is the place of GOD: Generator, Operator, Destroyer.
A custom synthesis should be easy given 5TFM-AMB'sclose relation with 5F-AMB.
The final step is militarization which given likely potency ought to be feasible.
Last edited: