Linear-Tailed Polyfluorinated Cannabinoids: Part 1
It's time to examine the Polyfluorinated Cannabinoids. This is a large topic so this first post is titled, "
Linear-Tailed Polyfluorinated Cannabinoids: Part 1"
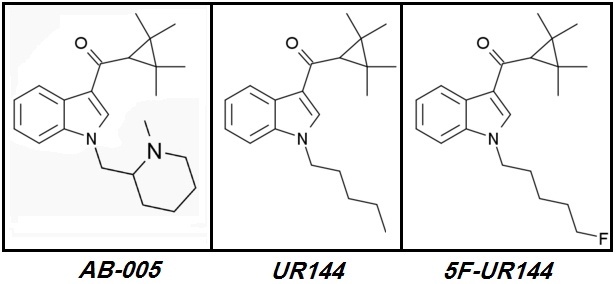
Let's start with the only Polyfluorinated Cannabinoid ever to reach the 'market':
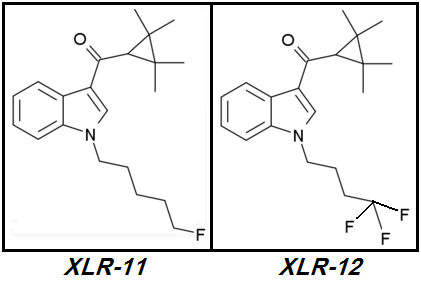
XLR-12.
XLR-12 (4TFM-UR144) [CAS# 895155-78-9](2,2,3,3-tetramethylcyclopropyl)[1-(4,4,4-trifluorobutyl)-1H-indol-3-yl]-methanone
XLR-12 was and continues to be a market failure. This Cannabinoid is named as
Compound 4 in the study below:
"In this study, we describe the identification of 19 newly distributed designer drugs, of which 8 were synthetic cannabinoids (1-8), 5 were cathinone derivatives (9-13), and 6 were other substances (14-19) (Fig. 1). In addition, we investigated the combination patterns of detected designer drugs, including compounds 1–19, in 104 illegal products purchased between November 2013 and May 2014."
XLR-12 never made it to widespread popularity despite its promising design.
XLR-12's
Brilliant designer even shortened
XLR-11's Pentyl tail to a Butyl in an obvious attempt to avoid what he saw as potential
excess Steric Hindrance due to Terminal Trifluorination.
He didn't remember that many very potent Cannabinoids such as the
CHMINACA &
FUBINACA series have bulky cyclic Tails which do not hinder their potency.
Two days ago I finally understood why
XLR-12 is such a failure: it's more expensive to manufacture than
XLR-11 however its potency is lower or no better than its monofluorinated parent. Why?
XLR-12's designer - likely a Chinese peon sitting behind a Dell Optiplex inside some nameless & likely grungy Chinese chemical factory - had the same
Brilliant Idea I had a week ago. The Brilliant Idea was for him and also for myself to increase the Tail's terminal electronegativity by further Fluoro-substitutions. Replace one or two of the last two Hydrogens on
XLR-11's terminal Fluoromethyl group with Fluorines.
He was as partly-mistaken as I was.
The single Fluoro-substitution of one Hydrogen atom thereby converting Cannabinoid Jekylls into their superpotent Hyde-versions works well for those below:
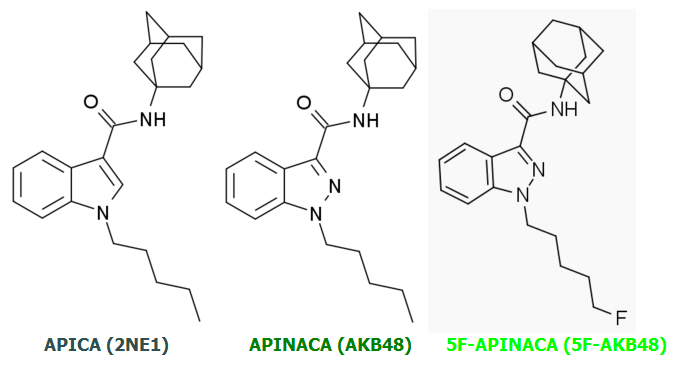
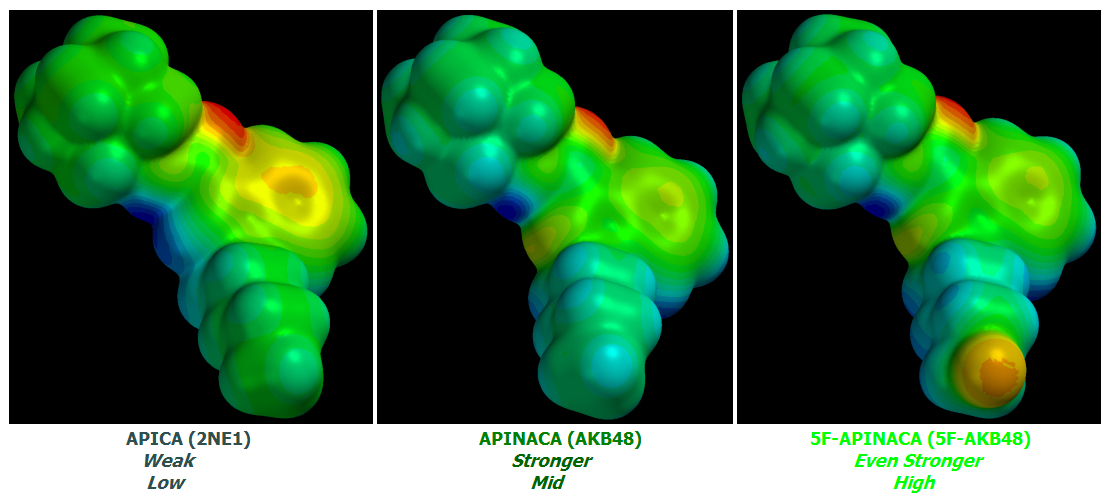
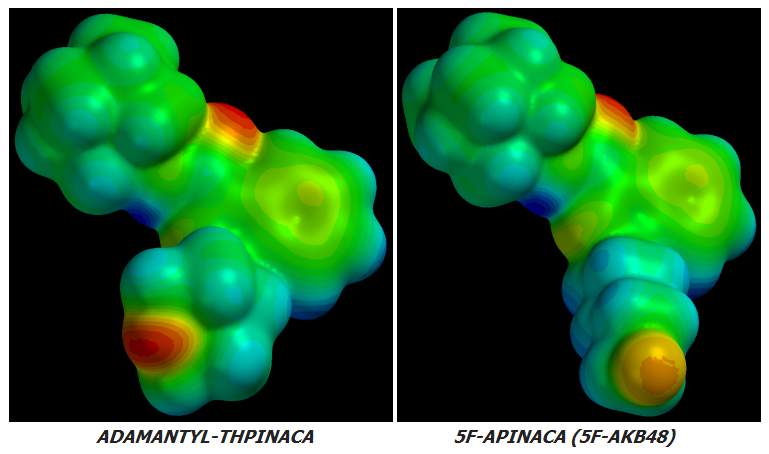
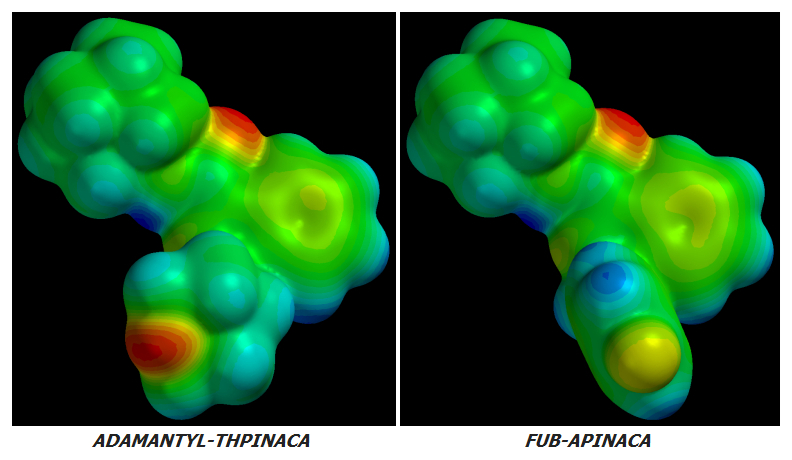
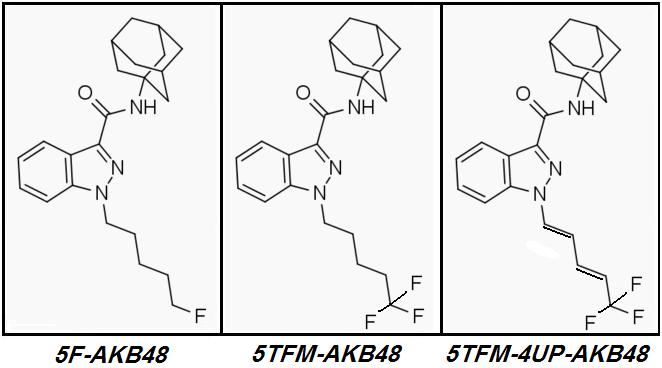
AKB48 => 5F-AKB48
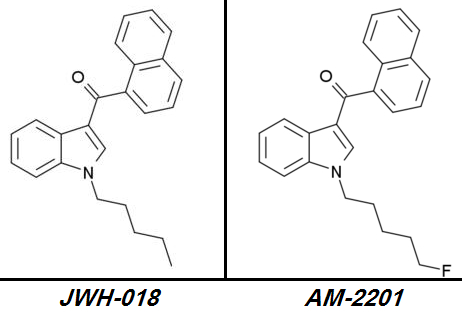
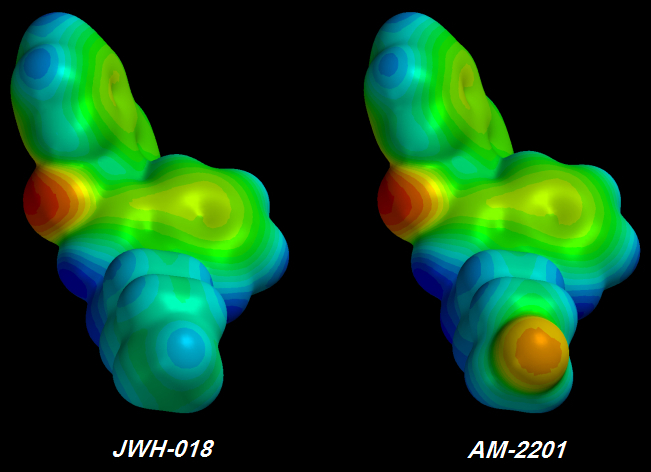
JWH-018 => AM-2201
UR-144 => 5F-UR144
In fact it ought to and likely does work well for any Cannabinoid of similar basic design.
However,
no further improvement in potency or CB1r:CB2r activation ratio can be achieved by further Fluoro-substitutions on either Alkane-tailed or Clyclohexyl-tailed Cannabinoids. At least not enough improvement to justify the increased cost of manufacture. The idea is sound in theory but its few real-world implementations have been failures in practice.
Electronegativity does not come from Fluorine atoms.
Fluorine atoms are thieves that steal electrons from other atoms & groups they're attached to because they're hungry for those electrons.
Thieves don't create wealth - they just redistribute it into their own greedy hands. And just like thieves Fluorine atoms cannot accumulate any more loot of Electrons stolen from other atoms
unless those atoms are both willing and able to give up Electrons. The Fluorine atom attached to the terminal Carbon in all of the successful Fluoro-Cannabinoids can steal Electrons from that Carbon and its attached Hydrogens. However, that is the limit to what it can steal because the rest of the chain is fully-saturated! It's an Alkane chain with each Carbon's electrons being fully-bound to two Hydrogens. Same goes for Cyclohexyl-tailed Cannabinoids.
Substituting a second or third Fluorine atom to in an attempt to
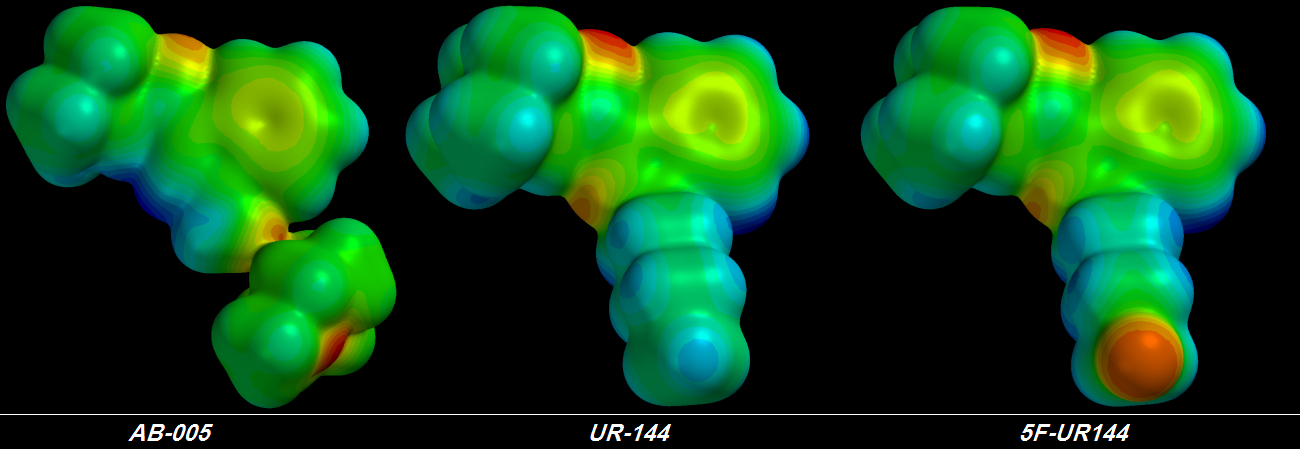
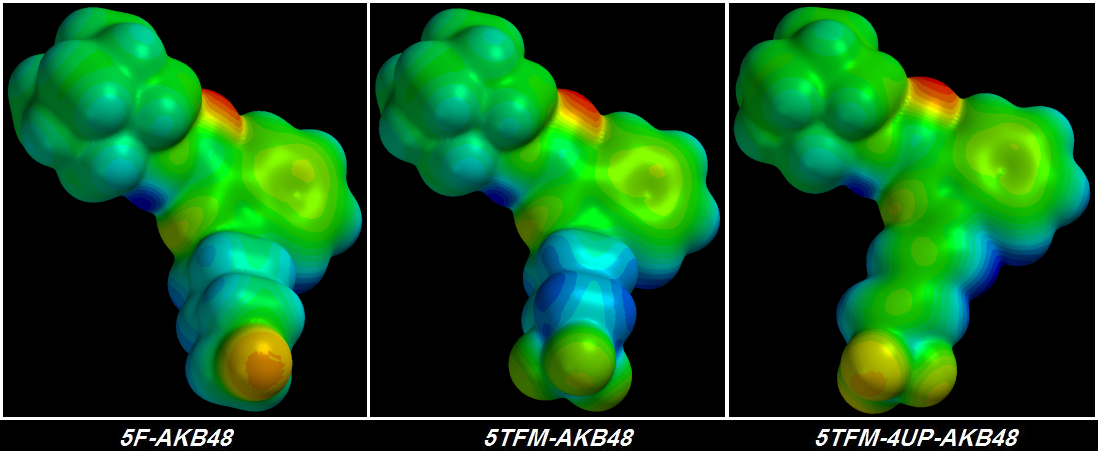
suck Electrons harder accomplishes nothing because no matter how hard those Fluorine atoms suck, the Electrons from the other atoms deeper in the chain are fixed into place tightly enough that they hardly move at all. I learned this lesson four days ago. Shivering & drooling with excitement, I plugged the first of my terminally-trifluorinated Cannabinoid molecules into the QED simulator. The final rendering was a sorry thing to behold.
Instead of a glorious giagantic group of three
[FLAMING RED] Fluorine atoms at the tail's tip I saw three green Fluorine atoms -
each of the three less electronegative than the original single [YELLOW] Fluorine atom. Furthermore, the chain behind the glorious pumped-up trifluorinated tip wasn't much bluer than before, indicating that no more Electrons could be stolen. It took me about a day to figure out the problem but I didn't want to believe it.
There is a way around this problem: Delocalize the Tail's Electrons:
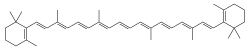
β-Carotene is a great and natural example of Electron delocalization:
Notice the alternating
Single-Double-Single-Double Carbon-Carbon bonds along
β-Carotene's chain? The magic is in the
pi Bonds [π bonds] which
allow Electrons to move along the chain instead of being fixed in one location. Delocalized.
What would happen if an Electron thief like Fluorine was substituted at the end of a chain whose structure allowed its electrons to easily move instead of being fixed in one place? I figure that the Fluorine atom could then suck and actually get more electrons. One Fluorine atom wouldn't get much more than its fill, but the case would be dramatically-different with two or three of them attached to the end. Then all the greedy Fluorines attached to the tip could
drink their fill of Electrons without just stealing them from each other. After all, the poor terminal Carbon in the existing Alkane-Tailed Cannabinoids has already been stolen from so much that it has become Destitute of Electrons. Riches to Rags with just one parasitical Fluorine atom attached to it like a Tapeworm or bloodsucking Flea.
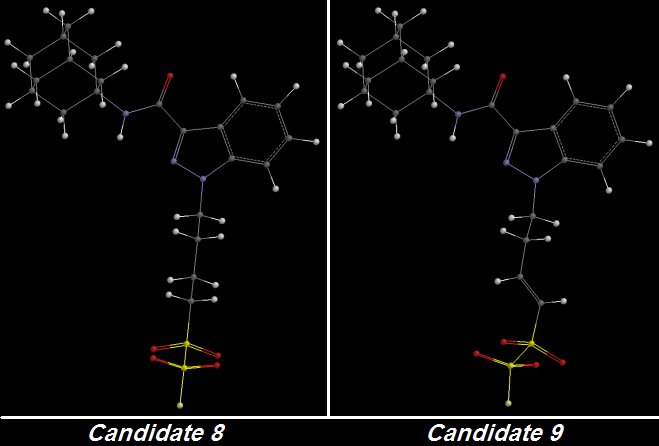
Candidates 8 and 9 are illustrative.
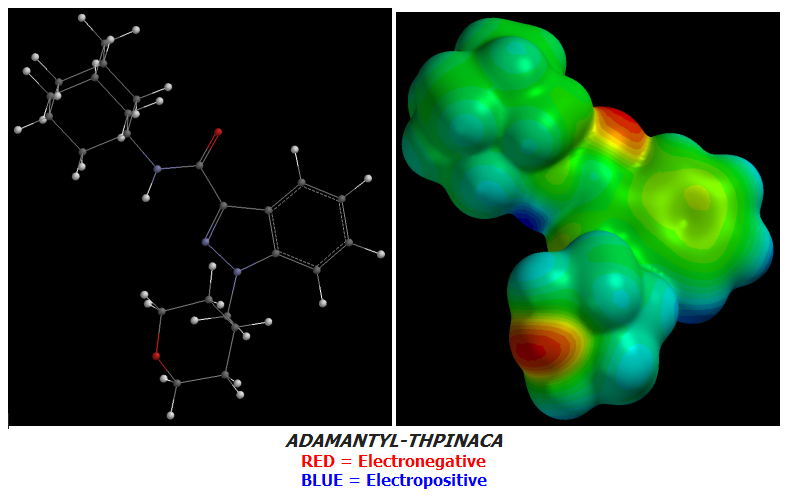
Candidate 8 is the second-last of my designs as of today and one that makes use of Oxygen atoms instead of Fluorines for the purpose of increasing terminal Electronegativity as ADAMANTYL-THPINACA does but with one exception: a single Fluorine atom at the very terminus to use up one final bond.
My design incorporates four Oxygens - two linked to each Sulfur atom - in a very compact terminal structure that has high Lipophilicity and has the potential [
no pun intended] for high Electronegativity:
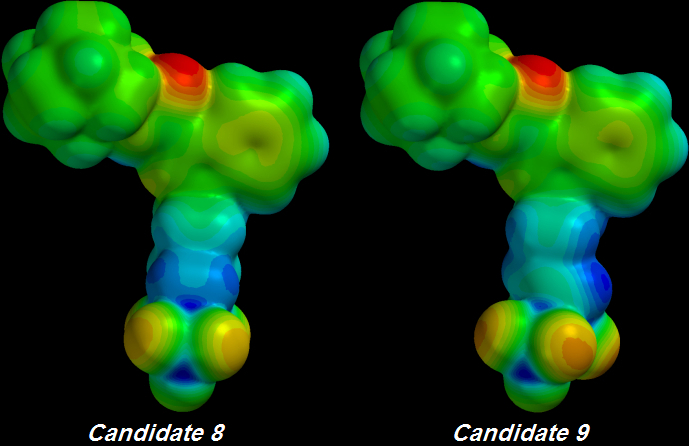
One C-C bond was unsaturated to create Candidate 9 from Candidate 8. That bond is not even directly connected to the first Sulfur atom but nonetheless it delocalizes enough electron pressure to increase the greedy terminal Oxygens' Electronegativity:
Candidate 9's Oxygens are
[DEEP ORANGE] rather than
Candidate 8's
[LIGHT ORANGE] colour.
Candidate 9's
[BLUER] Tail indicates that electrons are now being withdrawn from one atom deeper toward the Core along its Tail.
The gimmicky
Parafluorodisulfonyl tip was designed to suck electrons as hard as possible and was marginally more successful than previous Candidates' Polyfluoro tips.
Tomorrow we will find out what happens to more 'normal' Sulfur-free mono- and poly-Fluorinated Cannabinoids when their tales are unsaturated.
Terminal Electronegativity is not likely to increase much in Monofluorinated cases but is expected to show significant increases in Polyfluorinated molecules.