Hi bluelighters
First of all i would like to tell you guys I LOVE downers (GABA), I have tried different drugs (GABA) such as ethanol, benzoes (no drunkieness), carisoprodol, meprobatmate (no drunkieness), 2-methyl-2-butanol, GHB, methaqualone ("BOSS" from S.A) and so on. But I only do drugs a few times a month.
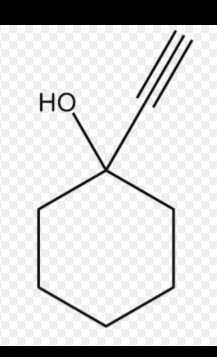
I have recently acquired 1kg of +99% 1-ethynyl-1-cyclohexanol, and would like to make a log because I don't seem to find MUCH info on here about it.
The drug is solid, but it is not like powder, it looks more like crystals or firbres.
I took 5g of the material and placed it in a glass-cylinder and then put the glass-cyinder in VERY hot water (boiling water) and in seconds it "melted" into liquid (about 5ml).
It does not smell as bad as 2M2B (and the aftertaste is not as bad).
My stats:
M23 l 173cm (5feet'8inch) l 73kg (160 pounds)
LOG (I have been fasting from 18:00):
21:00 l I took 0.1ml to see if any allergic reaction would be present
21:50 l There were no effect, so I took 0.5ml
22:30 l Felt some mild anxiolytic effect therefor I took 0.5ml more
23:45 l No "drunkiness" yet, but only more powerful anxiolytic effect so I took 0.6ml more.
00:50 l Took 0.5ml more
01:45 l Seems a bit intoxicated but not enough, so took 0.5ml more
02:35 l Feels nice, a bit uninhibited and wants to be at a club/bar or something but took 0.5ml more.
03:10 l I feel i little bit drunk but I can still walk "normally" so took 0.5ml more
03:50 l Now i started to feel dizzy and can't think clearly, and I have to close one eye to see whats on the pc-screen and I can't walk properly now, it is about here i like to be.
I feel very tiered and euphoric. I now start to feel the "need" for stimulants (cocaine or speed, as I usually do when doing too high amounts of EtOH or 2M2B)
= TOTAL 3.7ml - BP and HR sayed about the same in all these hours.
i guess 3.2ml would have been enough for me for feeling "good" and social/partymode.
(this MIGHT be a high dose for some, but I have very high tolerance for some drugs, I don't know why, but I only get pissed in the weekends and not even very weekend, it is just my brain chemistry i guess, BTW I cant feel subtle drugs like phenibut and modafinil) I usually do 15-20ml 2M2B so "normal" guys should properly only do half of the dose i did, first time.
I only combined this stuff with Coca-Cola and cigarettes (uses both daily)
This stuff is nice. NO nausea, NO stomach discomfort, and NO bad smell on breath (only a little menthol smell) like 2M2B.
Tomorrow I write here if I get a bad hangover, if NOT this is a great substance.
I have seen some guys on here write about this stuff being bad for the CYP450 enzyme, but I take that with a grain of salt, see all the people who have used this stuff medically (Ethinamate) daily with no bad effect and the people who have used Ethchlorvynol (a triple bound alcohol like this) daily with no ill-effects. So I don't think this stuff is "bad" even though it got ethynyl.
Please come with your inputs.
First of all i would like to tell you guys I LOVE downers (GABA), I have tried different drugs (GABA) such as ethanol, benzoes (no drunkieness), carisoprodol, meprobatmate (no drunkieness), 2-methyl-2-butanol, GHB, methaqualone ("BOSS" from S.A) and so on. But I only do drugs a few times a month.
I have recently acquired 1kg of +99% 1-ethynyl-1-cyclohexanol, and would like to make a log because I don't seem to find MUCH info on here about it.
The drug is solid, but it is not like powder, it looks more like crystals or firbres.
I took 5g of the material and placed it in a glass-cylinder and then put the glass-cyinder in VERY hot water (boiling water) and in seconds it "melted" into liquid (about 5ml).
It does not smell as bad as 2M2B (and the aftertaste is not as bad).
My stats:
M23 l 173cm (5feet'8inch) l 73kg (160 pounds)
LOG (I have been fasting from 18:00):
21:00 l I took 0.1ml to see if any allergic reaction would be present
21:50 l There were no effect, so I took 0.5ml
22:30 l Felt some mild anxiolytic effect therefor I took 0.5ml more
23:45 l No "drunkiness" yet, but only more powerful anxiolytic effect so I took 0.6ml more.
00:50 l Took 0.5ml more
01:45 l Seems a bit intoxicated but not enough, so took 0.5ml more
02:35 l Feels nice, a bit uninhibited and wants to be at a club/bar or something but took 0.5ml more.
03:10 l I feel i little bit drunk but I can still walk "normally" so took 0.5ml more
03:50 l Now i started to feel dizzy and can't think clearly, and I have to close one eye to see whats on the pc-screen and I can't walk properly now, it is about here i like to be.
I feel very tiered and euphoric. I now start to feel the "need" for stimulants (cocaine or speed, as I usually do when doing too high amounts of EtOH or 2M2B)
= TOTAL 3.7ml - BP and HR sayed about the same in all these hours.
i guess 3.2ml would have been enough for me for feeling "good" and social/partymode.
(this MIGHT be a high dose for some, but I have very high tolerance for some drugs, I don't know why, but I only get pissed in the weekends and not even very weekend, it is just my brain chemistry i guess, BTW I cant feel subtle drugs like phenibut and modafinil) I usually do 15-20ml 2M2B so "normal" guys should properly only do half of the dose i did, first time.
I only combined this stuff with Coca-Cola and cigarettes (uses both daily)
This stuff is nice. NO nausea, NO stomach discomfort, and NO bad smell on breath (only a little menthol smell) like 2M2B.
Tomorrow I write here if I get a bad hangover, if NOT this is a great substance.
I have seen some guys on here write about this stuff being bad for the CYP450 enzyme, but I take that with a grain of salt, see all the people who have used this stuff medically (Ethinamate) daily with no bad effect and the people who have used Ethchlorvynol (a triple bound alcohol like this) daily with no ill-effects. So I don't think this stuff is "bad" even though it got ethynyl.
Please come with your inputs.
Last edited: