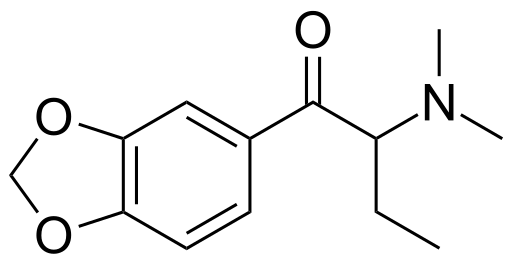
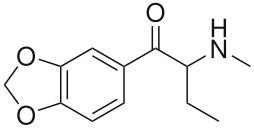
Is this what is meant by dibutylone? I can't think of any other way it could be intended, but then again vendors will name shit whatever, serotoni, benzo fury, rubbish all.
So naming, hmm. I still suck at IUPAC. You could think of this as 3,4-methylenedioxy-α,α-diethyl-β-keto-N-methylphenethylamine, which is so, so wrong, or 3,4-methylenedioxy-β-keto-α-ethyl-N-methylphenbutylamine, with diethylamine replacing isopropylamine, or instead 3,4-methylenedioxy-β-keto-N-methylaldibutamine, with aldibutylamine, or aladbutamine, used in the manner of
alpha
methyl
phene
thyl
amine = amphetamine, for aldibutylamine = alphadibutylphenethylamine or aladibutamine = alphalphadibutylphenethylamine but none of those are right. In fact they are all obscenely wrong.
Lemme take a sec and try to do the IUPAC.
Okay, I'm not sure how to handle the 'dibutyl' since that's ambiguous and means fuckall in this case (dibutylamine is a real thing but in this case that would be a tertiary amine of N-butyl-N-ethyl on a butyl backbone, to form the other butyl of 'butylamine'.. This is total nonsense so don't pay attention to what I just said). Dibutylone is just so, so fucking wrong of a name, which makes this weird since the structure may not even be correctly depicted above.
SO, assuming it IS pictured correctly, I'm gonna go with (this is anything but authoritative, is my best guess) 1-(1,3-benzodioxol-5-yl)-2-ethyl-2-(methylamino)butan-1-one.
People who can IUPAC all day long, am I anywhere close? My logic is its the usual 1,3-benzodioxole with the 5-yl to point out where on it the aliphatic chain meets. Then since the dibutyl (assuming I guessed the structure right in the picture above) means that the longest chain is still a butyl chain, so you get the butan part there. Then we have substitutions, a ketone, a methylamino, and the other 'dibutyl', which I'm guessing is actually an ethyl. So using priority rules for alphabetical starting letters you'd have 2-ethyl then 2-(methylamino) and then 1-one.
This brings up my original, unarticulated point. You can't have a 'dibutylamine' *as a structure linking the N and the phenyl/benzodioxole* in the way that you can have an isopropylamine or ethylamine, all substitutions aside. Dibutyl makes it sound like there's a whopping four more carbons atoms there, all unsaturated. So where would that go, to be properly called dibutyl?
Because butylone is named such since the *longest carbon chain* is butyl, but that is the backbone of the molecule. So either you'd have 'dibutyl) in the sense that there would be *two* butyls both coming off of that 5-yl of the benzodioxole, one being the ordinary aliphatic backbone holding the N-methyl amine and the other just being a whole other butyl just floating around, or else they may mean what I pictured, which I can think of as either alpha,alpha-diethyl-beta-keto-N-methylphenethylamine, which is IUPAC-incorrect since either ethyl can be part of the butyl that is the longest carbon chain there, or alpha-ethyl-beta-keto-alpha-(methylamino)butane, which I simplify to 1-ethyl-2-(methylamino)butan-1-one in alphabetical order...
Not that that's how you form IUPAC names, but the nomenclature common to PIHKaL uses the alpha, beta language and different, non-IUPAC terms. Like PIHKaL nomenclature (yes i know Shulgin included the real names in the headings for each drug in book 2 but people dont use those most of the time here in PD) would be 3,4-methylenedioxy-beta-keto-N-methylamphetamine for methylone instead of 2-methylamino-1-(3,4-methylenedioxyphenyl)propan-1-one.
Anyway does anybody see why I find this naming stupid? And did I royally butt-fuck the attempt at IUPACing the name?