nuke
Bluelighter
- Joined
- Nov 7, 2004
- Messages
- 4,191
"Yo dawg, I heard you like phenethylamines, so I put some phenethylamines on your tryptamines so you can trip while you trip!"
Ph.D. Thesis is entitled, "Tryptamines as Ligands and Modulators of the Serotonin 5-HT2A Receptor and the Isolation of Aeruginascin from the Hallucinogenic Mushroom Inocybe aeruginascens." By Niels Jensen, from die Georg-August-Universität zu Göttingen.
There are some interesting 5HT2A agonists reported, aside from a vast array of 5HT2A antagonists. Activity (EC50/IA) is measured from PI turnover. I'll just report the ones that show at least partial agonist activity below 1 uM. I haven't seen this thesis discussed often, so I thought I'd throw out this data for everyone.
IA = Intrinsic activity
5-Unsubstituted Tryptamine 5HT2A Partial Agonists
REFERENCE: DMT 41% IA at 2239 nM
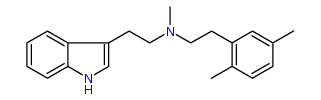
N-Methyl-N-(2,5-dimethyl)phenylethyltryptamine 26% IA at 235 nM, 112% IA at 32 uM
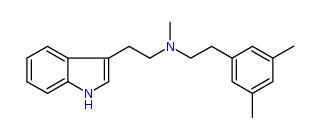
N-Methyl-N-(3,5-dimethyl)phenylethyltryptamine 41% IA at 281 nM, 86% IA at 10 uM, 164% IA at 32 uM
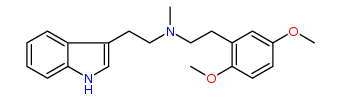
N-Methyl-N-(2,5-dimethoxy)phenylethyltryptamine 19% IA at 18 nM, 37% IA at 10 uM
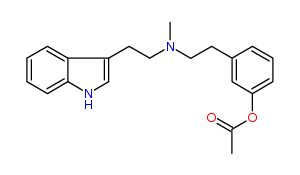
N-Methyl-N-(3-acetoxy)phenylethyltryptamine 38% IA at 98 nM, 57% IA at 10 uM, 92% IA at 32 uM
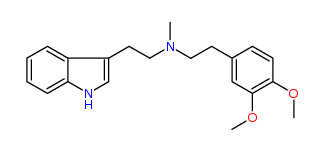
N-Methyl-N-(3,4-dimethoxy)phenylethyltryptamine 34% IA at 44 nM, 42% IA at 10 uM
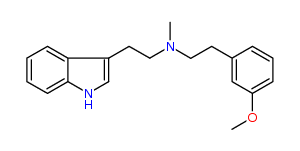
N-Methyl-N-(3-methoxy)phenylethyltryptamine 28% IA at 324 nM, 43% IA at 10 uM, 92% IA at 32 uM
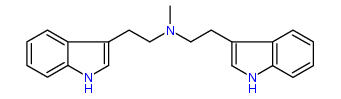
N-Methyl-N-(3-indolyl)ethyltryptamine 25% IA at 132 nM, 30% IA at 10 uM, 54% IA at 32 uM
N-Methyl-N-(3-(5-MeO)indolyl)ethyltryptamine 24% IA at 50 nM, 30% IA at 10 uM, 54% IA at 32 uM
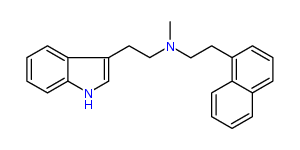
N-Methyl-N-1-naphthyltryptamine 26% IA at 290 nM, 64% IA at 10 uM, 94% IA at 32 uM
5-Methoxylated Tryptamine 5HT2A Partial Agonists
REFERENCE: 5-MeO-DMT 98% IA at 741 nM
N-Methyl-N-(2,5-dimethyl)phenylethyl-5-MeO-tryptamine 34% IA at 309 nM
N-Methyl-N-(2,5-dimethoxy)phenylethyl-5-MeO-tryptamine 27% IA at 320 nM, 31% IA at 10 uM, 47% IA at 32 uM
N-Methyl-N-(3,4-dimethoxy)phenylethyl-5-MeO-tryptamine 62% IA at 84 nM, 75% IA at 10 uM
N-Methyl-N-(4-methoxy)phenylethyl-5-MeO-tryptamine 40% IA at 302 nM, 27% IA at 1 uM (what? bimodal?), 42% IA at 10 uM
N-Methyl-N-(3-indolyl)ethyl-5-MeO-tryptamine 24% IA at 50 nM, 30% IA at 10 uM, 54% IA at 32 uM
N-Methyl-N-(3-(5-MeO)indolyl)ethyl-5-MeO-tryptamine 64% IA at 87 nM, 79% IA at 32 uM
N-Methyl-N-1-naphthyl-5-MeO-tryptamine 14% IA at 182 nM, 46% IA at 10 uM
N-Methyl-N-(3,4,5-dimethoxy)phenylpropyl-5-MeO-tryptamine 47% IA at 257 nM, 57% IA at 32 uM
N-Methyl-N-allyl-5-MeO-tryptamine 92% IA at 575 nM
N-Methyl-N-propynyl-5-MeO-tryptamine 81% IA at 811 nM, 93% IA at 32 uM
Other notable stuff:
- The 5-MeO-T derivatives appear to lose their crazy affinity for 5HT1A as compared to 5HT2A (5-MeO-DMT is almost 5 fold more selective for 5HT1A as compared to DMT), except for N-Methyl-N-(3,4,5-dimethoxy)phenylpropyl-5-MeO-tryptamine, N-Methyl-N-allyl-5-MeO-tryptamine, and N-Methyl-N-(3-(5-MeO)indolyl)ethyl-5-MeO-tryptamine. I will conjecture that they're probably more subjectively DMT or 4-HO-DMT like if they are active, but who knows.
- The 5-Unsubstituted tryptamine derivatives completely lose selectivity for 5HT1A over 5HT2A, with an order of magnitude or more loss in 5HT1A:5HT2A Ki ratio
- Both the 5-MeO-T derivatives and the 5-unsubstituted tryptamine derivatives tend to have smaller 5HT2A:5HT2C binding ratios as compared to their reference N,N-dimethylated psychedelics; the significance of this is unknown
Ph.D. Thesis is entitled, "Tryptamines as Ligands and Modulators of the Serotonin 5-HT2A Receptor and the Isolation of Aeruginascin from the Hallucinogenic Mushroom Inocybe aeruginascens." By Niels Jensen, from die Georg-August-Universität zu Göttingen.
There are some interesting 5HT2A agonists reported, aside from a vast array of 5HT2A antagonists. Activity (EC50/IA) is measured from PI turnover. I'll just report the ones that show at least partial agonist activity below 1 uM. I haven't seen this thesis discussed often, so I thought I'd throw out this data for everyone.
IA = Intrinsic activity
5-Unsubstituted Tryptamine 5HT2A Partial Agonists
REFERENCE: DMT 41% IA at 2239 nM
N-Methyl-N-(2,5-dimethyl)phenylethyltryptamine 26% IA at 235 nM, 112% IA at 32 uM
N-Methyl-N-(3,5-dimethyl)phenylethyltryptamine 41% IA at 281 nM, 86% IA at 10 uM, 164% IA at 32 uM
N-Methyl-N-(2,5-dimethoxy)phenylethyltryptamine 19% IA at 18 nM, 37% IA at 10 uM
N-Methyl-N-(3-acetoxy)phenylethyltryptamine 38% IA at 98 nM, 57% IA at 10 uM, 92% IA at 32 uM
N-Methyl-N-(3,4-dimethoxy)phenylethyltryptamine 34% IA at 44 nM, 42% IA at 10 uM
N-Methyl-N-(3-methoxy)phenylethyltryptamine 28% IA at 324 nM, 43% IA at 10 uM, 92% IA at 32 uM
N-Methyl-N-(3-indolyl)ethyltryptamine 25% IA at 132 nM, 30% IA at 10 uM, 54% IA at 32 uM
N-Methyl-N-(3-(5-MeO)indolyl)ethyltryptamine 24% IA at 50 nM, 30% IA at 10 uM, 54% IA at 32 uM
N-Methyl-N-1-naphthyltryptamine 26% IA at 290 nM, 64% IA at 10 uM, 94% IA at 32 uM
5-Methoxylated Tryptamine 5HT2A Partial Agonists
REFERENCE: 5-MeO-DMT 98% IA at 741 nM
N-Methyl-N-(2,5-dimethyl)phenylethyl-5-MeO-tryptamine 34% IA at 309 nM
N-Methyl-N-(2,5-dimethoxy)phenylethyl-5-MeO-tryptamine 27% IA at 320 nM, 31% IA at 10 uM, 47% IA at 32 uM
N-Methyl-N-(3,4-dimethoxy)phenylethyl-5-MeO-tryptamine 62% IA at 84 nM, 75% IA at 10 uM
N-Methyl-N-(4-methoxy)phenylethyl-5-MeO-tryptamine 40% IA at 302 nM, 27% IA at 1 uM (what? bimodal?), 42% IA at 10 uM
N-Methyl-N-(3-indolyl)ethyl-5-MeO-tryptamine 24% IA at 50 nM, 30% IA at 10 uM, 54% IA at 32 uM
N-Methyl-N-(3-(5-MeO)indolyl)ethyl-5-MeO-tryptamine 64% IA at 87 nM, 79% IA at 32 uM
N-Methyl-N-1-naphthyl-5-MeO-tryptamine 14% IA at 182 nM, 46% IA at 10 uM
N-Methyl-N-(3,4,5-dimethoxy)phenylpropyl-5-MeO-tryptamine 47% IA at 257 nM, 57% IA at 32 uM
N-Methyl-N-allyl-5-MeO-tryptamine 92% IA at 575 nM
N-Methyl-N-propynyl-5-MeO-tryptamine 81% IA at 811 nM, 93% IA at 32 uM
Other notable stuff:
- The 5-MeO-T derivatives appear to lose their crazy affinity for 5HT1A as compared to 5HT2A (5-MeO-DMT is almost 5 fold more selective for 5HT1A as compared to DMT), except for N-Methyl-N-(3,4,5-dimethoxy)phenylpropyl-5-MeO-tryptamine, N-Methyl-N-allyl-5-MeO-tryptamine, and N-Methyl-N-(3-(5-MeO)indolyl)ethyl-5-MeO-tryptamine. I will conjecture that they're probably more subjectively DMT or 4-HO-DMT like if they are active, but who knows.
- The 5-Unsubstituted tryptamine derivatives completely lose selectivity for 5HT1A over 5HT2A, with an order of magnitude or more loss in 5HT1A:5HT2A Ki ratio
- Both the 5-MeO-T derivatives and the 5-unsubstituted tryptamine derivatives tend to have smaller 5HT2A:5HT2C binding ratios as compared to their reference N,N-dimethylated psychedelics; the significance of this is unknown