Mugz
Bluelighter
- Joined
- Apr 6, 2004
- Messages
- 15,449
Early test data appears to show in-vitro monoamine affinities and ratios somewhere in between MDPV and Desoxypipradrol, but with much relief (given the lack of spare time to spend in the lab) it appears the in-vitro half life is maybe only 4 hours.
Anyone heard anything else about this chem, or have any first hand experience. Would also appreciate comments from the pharmacologists that we have around the site that could comment on whether or not it is theoretically safe.
Wikipedia Entry
PubChem Page
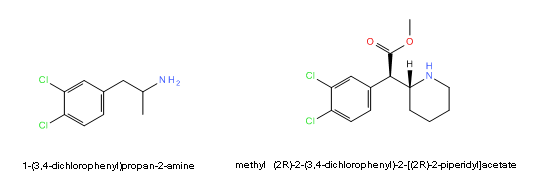
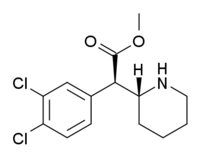
methyl (2R)-2-(3,4-dichlorophenyl)-2-[(2R)-piperidin-2-yl]acetate, 3,4-dichloro-methylphenidate, 3,4-CTMP, 3,4-DCMP, CTMP, DCMP, dichloromethylphenidate
Anyone heard anything else about this chem, or have any first hand experience. Would also appreciate comments from the pharmacologists that we have around the site that could comment on whether or not it is theoretically safe.

Wikipedia Entry
PubChem Page
methyl (2R)-2-(3,4-dichlorophenyl)-2-[(2R)-piperidin-2-yl]acetate, 3,4-dichloro-methylphenidate, 3,4-CTMP, 3,4-DCMP, CTMP, DCMP, dichloromethylphenidate
Last edited by a moderator: