First SAR rule of all: SAR does not carry over from one sort of drug to a relatively unrelated other drug. There is no reason why they would respond in the same way to a subtitution unless you know that they bind to a receptor in some same way spatially.
Remember the primitive lock and key analogy for drugs in receptors and realize which drugs actually act on which receptors and whether it actually makes sense to try and make a key that fits well in all.
Unfortunately even drugs that seem related in certain ways may turn out to bind upside down to a receptor compared to each other... this again would ruin any plans you might have had to make a hybrid that bridges the 'gap'. What you need is two drugs that behave similarly and even bind similarly, so that you can enhance that binding with a well-placed chemical group. There is often little tolerance for what sort of group must be or can be placed somewhere to make a drug better... ahh if it were only that easy right? No drug designers really put the art in 'the art of science' i guess... although that stuff *can* be simulated and calculated etc..
So hybridizing two drugs is really very rare and afaik only tends to happen sometimes with drugs that share *some* pharmacology and even then it is a tradeoff: you are basically making one type of drug worse at something by trying to turn it more into another one.
Think of breeding animals... you got your liger for example, but there are quickly limits you encounter trying to breed two types of animals that aren't that related like a parrot and a crocodile, and even if you could do that - it would be a lottery winner if it would then have a fighting chance in nature let alone if it can survive in the local environment where you want to keep it as a pet (you sadist bastard). Not too good an analogy [what about genetic engineering / modification for example?] well that is screwing with the chemistry at a another level.. with drugs you are already at that deepest level.
MDMA is not super related to cocaine, it's an empathogen and acts promiscuously on triple monoamines in various ways including as releaser while cocaine is a reuptake inhibitor with a preference for dopamine.
Since cocaine doesn't act as releaser that way, you can't really enhance that hypothetical activity to make it more MDMA-like, at least not simply that way...
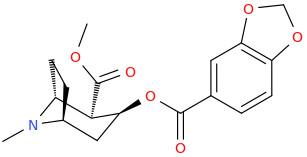
There are 4'-subtituted analogues:
https://en.wikipedia.org/wiki/List_of_cocaine_analogues#para-substituted_benzoylmethylecgonines
And looking at these my best guess would be that the above molecule may retain some activity as DRI although possibly not very good.. but who knows it is not enough info to really go on..
This looks more like a supercharged coke:
https://en.wikipedia.org/wiki/Salicylmethylecgonine
But hey it wouldn't be the first coke analogue that sounds amazing on paper but sucks ass according to people who try it - like worse than inactive the way I read it.
Talk to nagelfar, he has been posting cocaine analogues like a coke-fuelled message posting rabbit, but I don't know if he has relevant info on this topic. Just wait here for everyone else to chime in too though.
Also: if you check out the way cocaine docks into DAT for example, you can sort of check out what amino acid residues are around that para position, what might be a better way to hang on to the cocaine molecule, where there is tolerance for substitution etc.
I don't know how e.g. MDPV docks but apparently that part of the drug is not involved with critical parts of it binding and activation. If it doesn't really matter much, as you put it, then on the other hand you also don't have much potential to supercharge your molecule with a modification and 'put it on steroids'.