-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
without going in to synth details would these be to hard to make and is that why we've not seen them?The binding site of methoxetamine is different from glutamate's, so it doesn't make sense to compare their structures.
4-keto group dramatically increases affinity to opioids receptors especially if there is a tertiary amine present in the molecule. However, one modification of the cyclohexane ring that boosts the activity at NMDA receptors is 2-methyl group, (-)-trans-2-Me-PCP (phenyl trans to Me) is 5x more potent than PCP in vitro and 2x more potent in vivo, so perhaps:

[MENTION=147526]Dresden[/MENTION] - install marvin beans, the pics are even better
https://i.imgur.com/MOMXoC0.png
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
They have two chiral centers (diastereomers), I would want to know if the other cis-2'-methyl (or 6' in the keto version?) enantiomers of those is any good, and especially not side-effect adding.. It wouldn't be that they are hard to make, but they are annoying to make as pure isomer and I don't see the industry taking a liking to such a complication.
It would be worthwhile if the cis enantiomers are nice or at worst harmless, so that the racemate - which shouldn't be very hard at all to make - suffices. In the case of e.g. 3,4-CTMP it worked out..
It would be worthwhile if the cis enantiomers are nice or at worst harmless, so that the racemate - which shouldn't be very hard at all to make - suffices. In the case of e.g. 3,4-CTMP it worked out..
adder
Bluelighter
- Joined
- Mar 28, 2006
- Messages
- 2,852
(-)-trans-2-Me-PCP is twice as potent as PCP and four times as potent as (+)-trans-2-Me-PCP (in vivo), so the site is sensitive to stereochemistry (though with racemic 2-Me-PCP you don't lose any potency vs. PCP ). It doesn't seem hard at all to obtain the more potent enantiomer in high purity, the trans racemate is separated from the cis racemate by plain crystallization and then respective enantiomers are obtained via chiral resolution.
). It doesn't seem hard at all to obtain the more potent enantiomer in high purity, the trans racemate is separated from the cis racemate by plain crystallization and then respective enantiomers are obtained via chiral resolution.
Here's the abstract of the original paper.
I myself like clean and elegant structures, ChemDraw gives one of the cleanest structures I've seen, ChemDoodle looks nice and clean as well. I used to use ChemSketch as I got used to it and thought drawing in ChemDraw was uncomfortable, but then I changed my mind and now I think it's more practical than ChemSketch. As for esthetics, ChemSketch's structures compared to ChemDraw's are like typescript compared to an elegant computer font. I don't like those coloured structures from Marvin Beans either. Also, with ChemDraw it's possible to export structures directly to .gif files, not to mention a lot of useful tools and simulations if you need them.
----

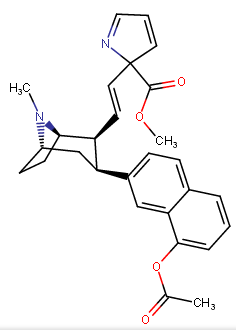
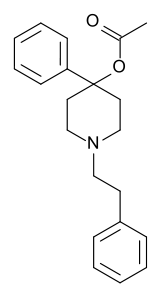
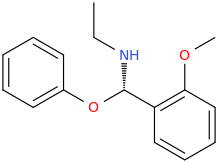
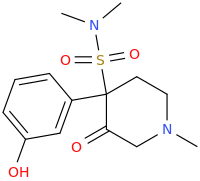
Let's call this one "eclectamine" and let's hope it'll live up to its name.
 ). It doesn't seem hard at all to obtain the more potent enantiomer in high purity, the trans racemate is separated from the cis racemate by plain crystallization and then respective enantiomers are obtained via chiral resolution.
). It doesn't seem hard at all to obtain the more potent enantiomer in high purity, the trans racemate is separated from the cis racemate by plain crystallization and then respective enantiomers are obtained via chiral resolution.Here's the abstract of the original paper.
@Dresden - install marvin beans, the pics are even better
I myself like clean and elegant structures, ChemDraw gives one of the cleanest structures I've seen, ChemDoodle looks nice and clean as well. I used to use ChemSketch as I got used to it and thought drawing in ChemDraw was uncomfortable, but then I changed my mind and now I think it's more practical than ChemSketch. As for esthetics, ChemSketch's structures compared to ChemDraw's are like typescript compared to an elegant computer font. I don't like those coloured structures from Marvin Beans either. Also, with ChemDraw it's possible to export structures directly to .gif files, not to mention a lot of useful tools and simulations if you need them.
----

Let's call this one "eclectamine" and let's hope it'll live up to its name.
Last edited:
roi
Bluelighter
- Joined
- Sep 2, 2013
- Messages
- 1,545
I don't like those coloured structures from Marvin Beans either.
View -> Colors -> Monochrome. And of course you can export structures into a variety of formats as well.
w0w0mg
Bluelighter
- Joined
- Dec 4, 2015
- Messages
- 848
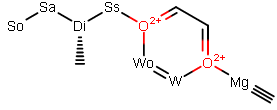
Can someone PLEASE make me 2 compounds,
one having the word "Sosadisso" and another saying "w0w0mg" in different places.
Example: So ----- Sa --- Di -Ss --O
Example: Wo --W-----O ---Mg
If someone could do that for me, I would love you all. lol.
one having the word "Sosadisso" and another saying "w0w0mg" in different places.
Example: So ----- Sa --- Di -Ss --O
Example: Wo --W-----O ---Mg
If someone could do that for me, I would love you all. lol.
Incunabula
Bluelighter
- Joined
- Dec 10, 2010
- Messages
- 1,862
I don't think the trans-2-Me-ketamine would be any good, I mean, of cause it could be, but not if you consider the fact, that going by the arylcyclohexylamines we know so far, ketamine seems to not follow the same SAR as the other Acha's.
It'll never work taking a substitution from a PCP analog, and then transfer it to ketamine. We'll never see a "better" version of ketamine, just legal less good ones imo. Anyway, the other ones are probably awesome though, especially the MXE one. Otherwise it's better to loose the chloro and then extend the n-methyl to ethyl and it's probably all rock'n'roll - or even better, loose the 2-ketone as well, to get Trans-2-Me-PCE.
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
Why do you say that? Ketamine was not developed as a recreational drug, so clearly they were looking for different qualities in their target compound.
If 2-fluoro-DCK is an interesting ketamine light, then who's to say that some or one of the various different subsitutions that may perhaps surface won't be an improvement? Subjectively of course.
But you may very well be right that borrowing from PCP SAR isn't predictive. Better to just compute 3D-QSAR and such for K mimics.
I wonder about 2-nitro-deschloroketamine, but with all aromatic nitro compounds there are potential dangers namely bone marrow depression and interactions with metabolic pathways (oxidative phosphorylation)...
If 2-fluoro-DCK is an interesting ketamine light, then who's to say that some or one of the various different subsitutions that may perhaps surface won't be an improvement? Subjectively of course.
But you may very well be right that borrowing from PCP SAR isn't predictive. Better to just compute 3D-QSAR and such for K mimics.
I wonder about 2-nitro-deschloroketamine, but with all aromatic nitro compounds there are potential dangers namely bone marrow depression and interactions with metabolic pathways (oxidative phosphorylation)...
Last edited:
adder
Bluelighter
- Joined
- Mar 28, 2006
- Messages
- 2,852
3-methoxy aryl substitution transferred well from PCP, it's the same site they're all interacting with, right? If the site can discriminate between dextrorotatory and levorotatory trans-2-Me-PCP, then in my opinion chances are the right diastereomer might benefit from 2-methyl group unless ketamine and methoxetamine bind in some drastically different way from PCP and PCE, which of course is possible.
BTW, I don't think that you can say with 100% certainty that we will not see a "better version" of ketamine. One thing is people perceive effects of drugs differently and two people enjoying ketamine effects might find another dissociative completely different. There won't be another ketamine, but there still may be an arylcyclohexanamine that will be as good as ketamine or methoxetamine, there's a still a great number of modifications and their combinations that can be applied to the backbone, so at this point statistically speaking there is a chance (well, always will be ).
).
BTW, I don't think that you can say with 100% certainty that we will not see a "better version" of ketamine. One thing is people perceive effects of drugs differently and two people enjoying ketamine effects might find another dissociative completely different. There won't be another ketamine, but there still may be an arylcyclohexanamine that will be as good as ketamine or methoxetamine, there's a still a great number of modifications and their combinations that can be applied to the backbone, so at this point statistically speaking there is a chance (well, always will be
Incunabula
Bluelighter
- Joined
- Dec 10, 2010
- Messages
- 1,862
Why do you say that?
It's just from thinking about the changes to ketamine's structure that we'we seen so far. And it seems to me, that ketamine has very unique effects compared to all the other available ones imo. Maybe it's just because it's duration is so short. I don't know, but I just think ketamine is so much more sharp, on edge and yes, more bizarre ( Yes, I know high dose everything is bizarre
So what have we tried with ketamine so far?
Remove the chloro? You get something thats more like MXE.
Change the chloro to something not halogen? you get inactive
Extend the n-ethyl? You get something that's nearly inactive, even though every other ArCHA benefits from that.
My point is, that all the other ArCHA's seem to have more in common SAR wise, than they do with ketamine. Because if some substitution or addition is great for PCP, it's probably also great for PCE and MXE and 3-MeO-PCP etc. But it doesn't mean that it's great for ketamine, because it's effects are still quite different. And ketamine just seems to be more finicky with changes. You quickly get something inactive or something which isn't related to ket at all in it's effects.
If 2-fluoro-DCK is an interesting ketamine light, then who's to say that some or one of the various different subsitutions that may perhaps surface won't be an improvement? Subjectively of course.
Yes, of cause I can't discount that. It just seems to me that neither fluoro nor bromo in the 2 position were improvements, from what I understand. Both were less potent, so bigger or smaller is a no go. TFM might be good, and hopefully we'll see about that at some point.
But were to go from there? Putting subs in other places on the phenyl is 100% to make something new, something very different, that's not going to be like ketamine at all - So in that sense, those analogs are just going to be new ArCHA's, not real ketamine analogs.
Not that there probably aren't things to try. You're just not going to get predictions about it from PCP analogs, because it just seems like a completely different world, no?
But you may very well be right that borrowing from PCP SAR isn't predictive. Better to just compute 3D-QSAR and such for K mimics.
Yes, exactly
Last edited:
Incunabula
Bluelighter
- Joined
- Dec 10, 2010
- Messages
- 1,862
3-methoxy aryl substitution transferred well from PCP, it's the same site they're all interacting with, right? If the site can discriminate between dextrorotatory and levorotatory trans-2-Me-PCP, then in my opinion chances are the right diastereomer might benefit from 2-methyl group unless ketamine and methoxetamine bind in some drastically different way from PCP and PCE, which of course is possible.
I didn't see that you posted while I was writing. I didn't mean to ignore you
Yes, I see what you mean, and of cause you might be right. As I wrote in my post above, I think there's reason to believe that ketamine interacts quite differently with the site, than any of the other ArCHA's (except fluoroketa and bromoketa and hopefully TFM-Keta) Of cause, it's just something I think, I have no evidence what so ever.
3-MeO-aryl seems wortwhile for more or less all constellations of ArCHA. But what if you put it on keta? 2-Cl-2-Oxo-3-MeO-PCM? A good guess is that it would be inactive. Now drop that 2-Cl and you'd have MXM, which is supposed to be like less potent, less spectacular MXE. Not that it doesn't have it's fans, but it says something about ketamine SAR imo.
BTW, I don't think that you can say with 100% certainty that we will not see a "better version" of ketamine. One thing is people perceive effects of drugs differently and two people enjoying ketamine effects might find another dissociative completely different. There won't be another ketamine, but there still may be an arylcyclohexanamine that will be as good as ketamine or methoxetamine, there's a still a great number of modifications and their combinations that can be applied to the backbone, so at this point statistically speaking there is a chance (well, always will be).
I totally agree. But I believe more in seeing a new MXE type drug in the future, than a new ketamine type drug, if you get my drift.
Definitely very fascinating with the whole trans-Me-PCP thing though
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
Can someone PLEASE make me 2 compounds,
one having the word "Sosadisso" and another saying "w0w0mg" in different places.
Example: So ----- Sa --- Di -Ss --O
Example: Wo --W-----O ---Mg
If someone could do that for me, I would love you all. lol.
For an avatar or something? What, like this? -
- Status
- Not open for further replies.