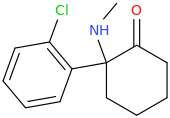
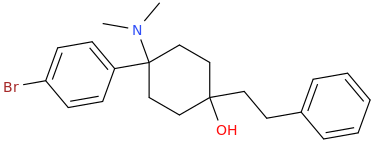
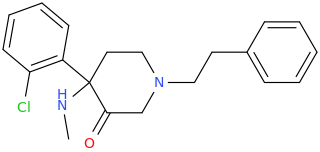
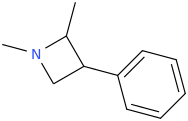
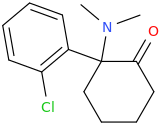
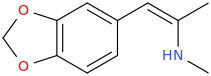
Wonder if this compound has any LSD-like activity?

Sumanirole
a D2 selective agonist (D2 agonists make rats(and humans!) incredibly horny!, is that right?

Sumanirole
a D2 selective agonist (D2 agonists make rats(and humans!) incredibly horny!, is that right?
Sumanirole (PNU-95,666) is a highly selective D2 receptor full agonist, the first of its kind to be discovered.[1][2][3] It was developed for the treatment of Parkinson's disease and restless leg syndrome. While it has never been approved for medical use [4][5] it is a highly valuable tool compound for basic research to identify neurobiological mechanisms that are based on a dopamine D2-linked (vs. D1, D3, D4, and D5-linked) mechanism of action. [3]