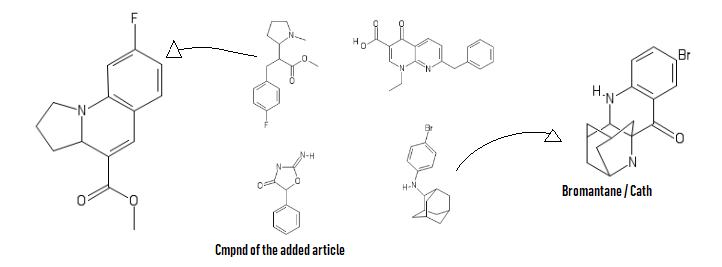
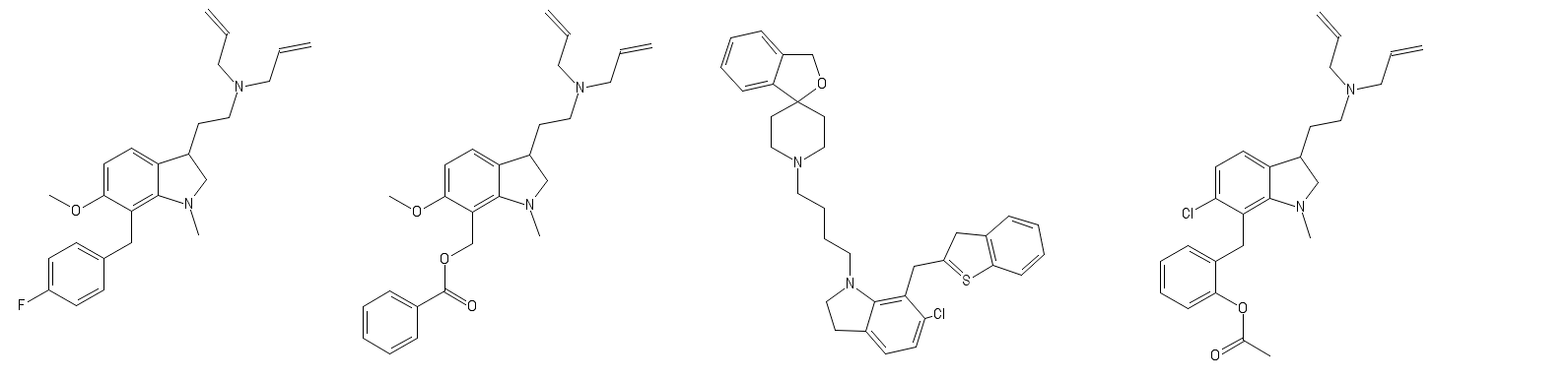
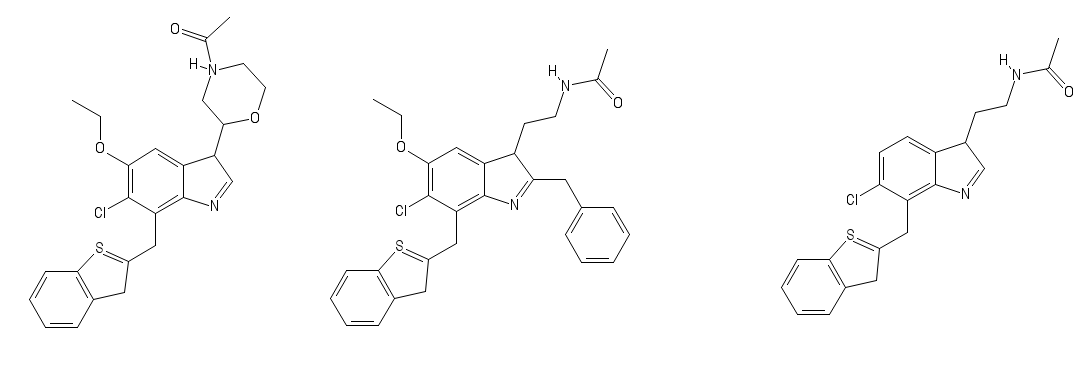
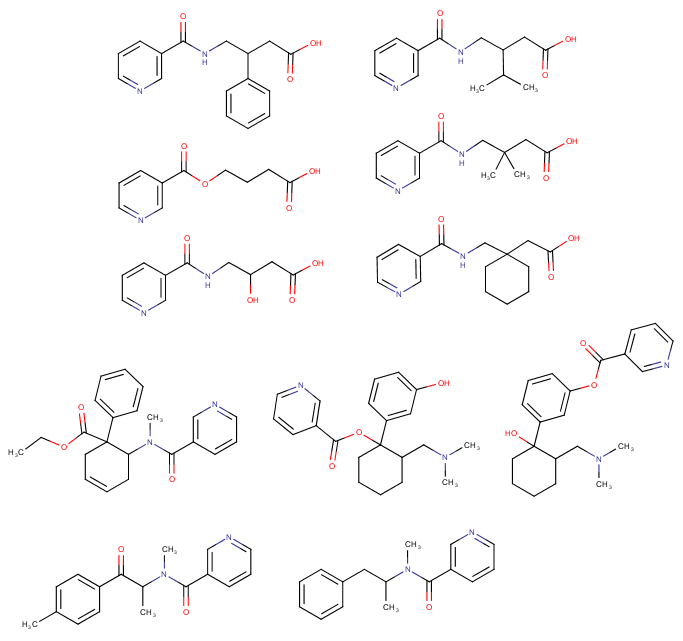
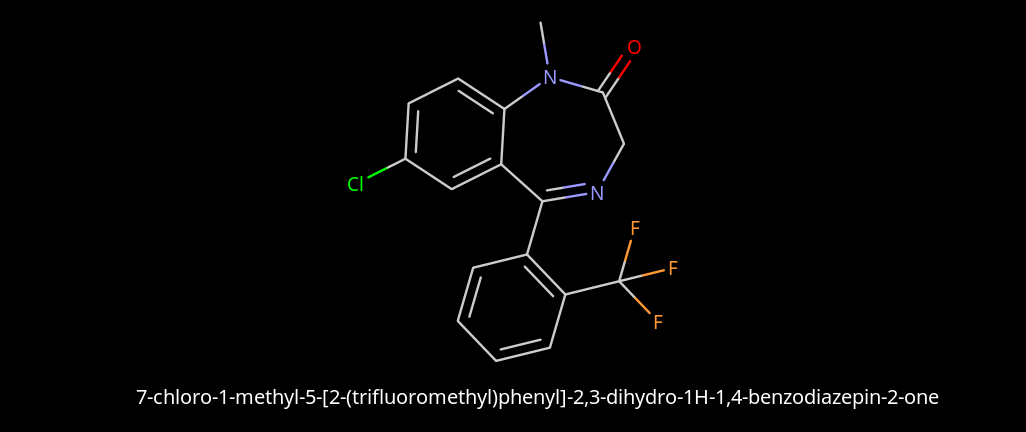
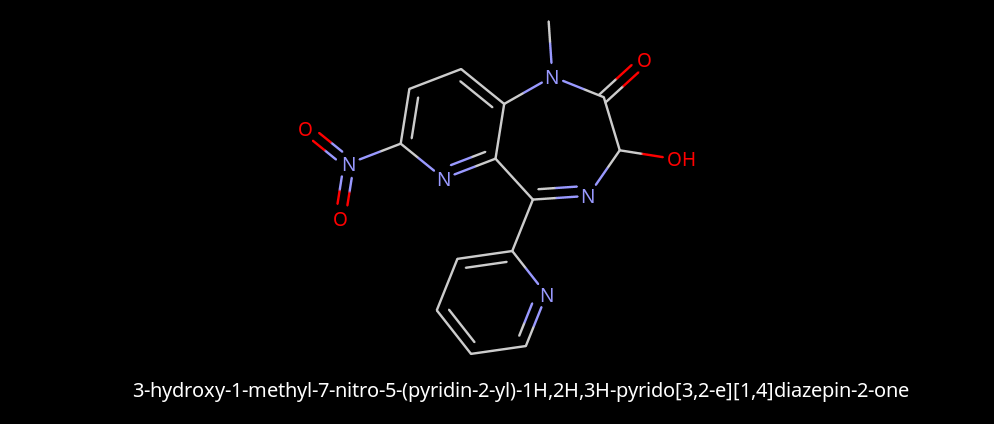
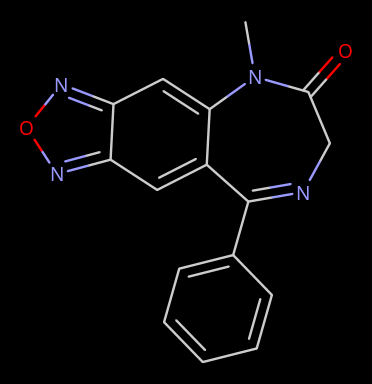
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
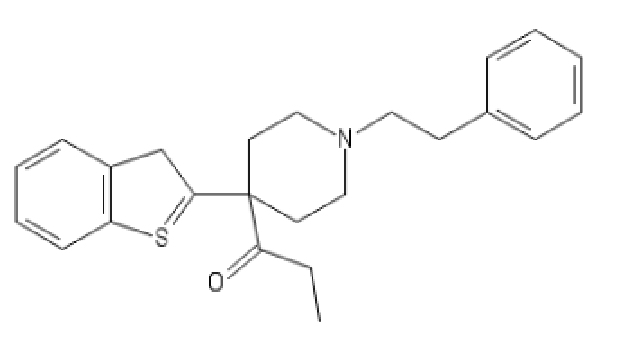
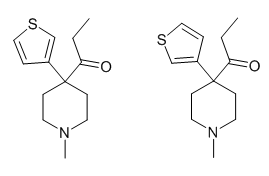
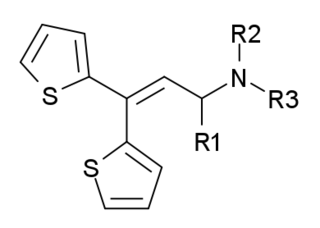
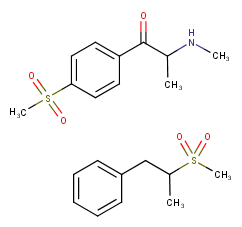
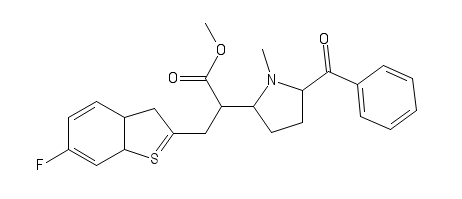
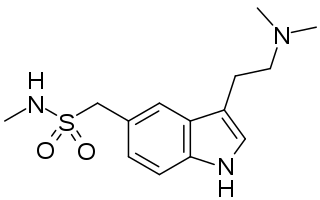
Dotchem-reminds me of a phenylated pethidine, with the nitrogen replaced with an ether bridge.
Not surprising that there is potential for both opioid effects and DARI properties.
That said...what is the potential for it's acting as a convulsant, as norpethidine can?
Another mixed DARI/MOR agonist that comes to mind, is etonitazene, and presumably the other benzimidazole opioids. Although etonitazine is a bit of a hot one to handle, from everything I've read of it. Apparently it surfaced at one point in russia, and was dosed by smoking, a cotton thread being soaked in a solution, and this poked into a cigarette with a needle (and presumably it'd be just as practical to roll your own, with the thread in place). For such a potent sonofabitch of an opioid, not a bad idea, as ideas concerning such potent opioids go to begin with, the slow administration in a cigarette allowing fine control over dosage.
Although the Highsmith case, using via nasal spray, that turned out pretty ugly.
Some of the lower potency benzimidazoles would be safer. Although don't they all have a really short duration of action?
Not surprising that there is potential for both opioid effects and DARI properties.
That said...what is the potential for it's acting as a convulsant, as norpethidine can?
Another mixed DARI/MOR agonist that comes to mind, is etonitazene, and presumably the other benzimidazole opioids. Although etonitazine is a bit of a hot one to handle, from everything I've read of it. Apparently it surfaced at one point in russia, and was dosed by smoking, a cotton thread being soaked in a solution, and this poked into a cigarette with a needle (and presumably it'd be just as practical to roll your own, with the thread in place). For such a potent sonofabitch of an opioid, not a bad idea, as ideas concerning such potent opioids go to begin with, the slow administration in a cigarette allowing fine control over dosage.
Although the Highsmith case, using via nasal spray, that turned out pretty ugly.
Some of the lower potency benzimidazoles would be safer. Although don't they all have a really short duration of action?