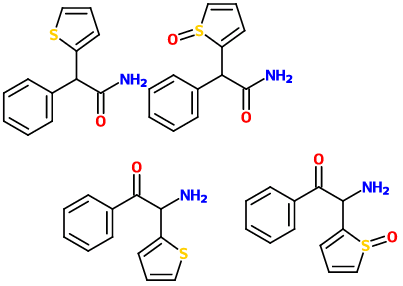
Why doesn't cathinone form imine polymers if they have ketones and primary amines? Is there something about the bulkiness preventing imine formation?
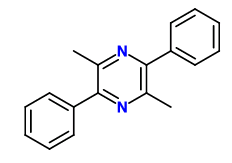
AFAIK, cathinone does condense/dimerize to 3,6-Dimethyl-2,5-Diphenylpyrazine, which is one of the reasons that khat needs to be eaten fresh (the other reason being the reduction of cathinone to the significantly less psychoactive cathine).
While the elimination of water should shift the equilibrium towards the condensation reaction, using the right methods the plant can apparently be dried without destroying most of the cathinone.




![(2S,5R,6R)-2-carbomethoxy-3,3-dimethyl-7-oxo-6-[(2-phenyl-1-methylethyl)amino]-4-thia-1-azabicyclo[3.2.0]heptane.png](/community/proxy.php?image=https%3A%2F%2Fopsin.ch.cam.ac.uk%2Fopsin%2F%282S%2C5R%2C6R%29-2-carbomethoxy-3%2C3-dimethyl-7-oxo-6-%5B%282-phenyl-1-methylethyl%29amino%5D-4-thia-1-azabicyclo%5B3.2.0%5Dheptane.png&hash=f7d8f734f1ccad1f96f3cd5a5116dc1e)