-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
Hey there guys so my dog was trying to complete the H Manufacturing process but is having trouble with the tar process and he's got everything to make the conversion with acetic anhydride and acetic acid glacial but can't make the conversion complete and doesn't know or can't find info on how to do it as he has the opium and the correct chemicals to make the process a good one for personal use still having trouble though you can't figure it out anybody can help with the molecule diagram and possibly the end process or process from A to B
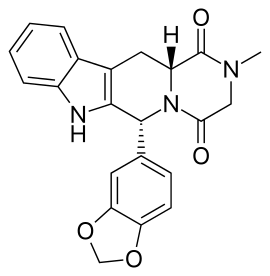
Are the methylene analogues of barbiturates active?
Diallylbarbiturate is active: see Allobarbital, although i don't like terminal alkenes, they look insidious
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
I know that much, barbiturates can play pretty fast and loose with the substituents, everything from methyl to isohexyl to phenyl is allowed. I was more interested in replacing the ureide group in barbiturates with either oxygens or methylenes.
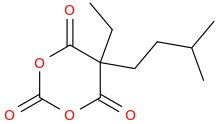
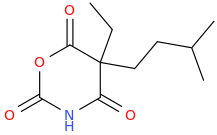
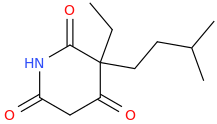
Glutethimide shares a similar structure:
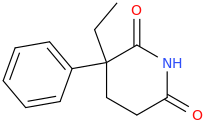



Glutethimide shares a similar structure:

Last edited:
sekio, i don't think the anhydride between [carbonic acid or carbamic acid] and malonic acid are stable to room temperature,
in the first case, i am not even sure if it can exists in dry ice cooled bath (-78C) without decomposition.
Third one looks VERY similar to glutethimide in regarding of shape and volume tho!
in the first case, i am not even sure if it can exists in dry ice cooled bath (-78C) without decomposition.
Third one looks VERY similar to glutethimide in regarding of shape and volume tho!
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
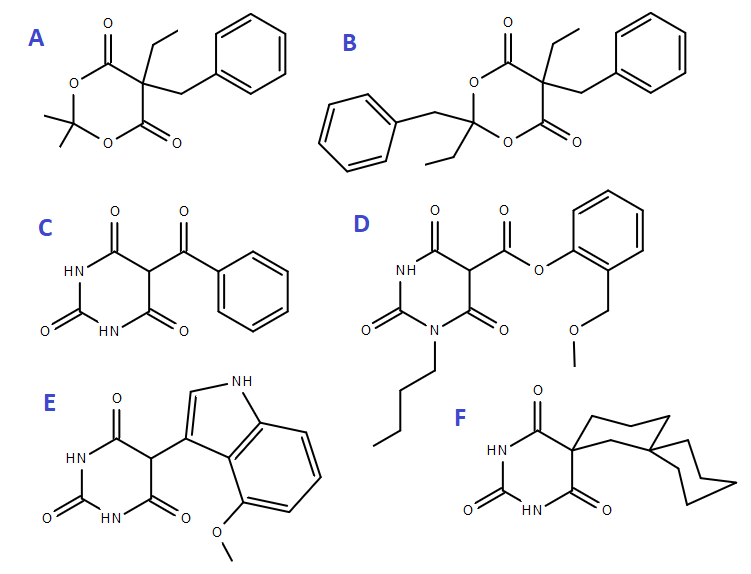
Can I get E but in a hydroxy instead of the methoxy.. or with a methoxy on the adjacent carbon? 
When I look at barbs I think: too bad, you could probably make em way more explosive.. (if this invites discussion on a topic which is inappropriate for the forum you can disregard this and I can delete this)
I have always found cyanuric acid a brilliant structure.. The tautomerism also applies to barbs. Not to mention cyanuric being essentially the compact perfect cyclic peptide haha.. The challenge is to switch from a super stable structure to an explosive or psychoactive one in the blink of an eye..
And also to form really sexy crystal lattices perhaps?
When I look at barbs I think: too bad, you could probably make em way more explosive.. (if this invites discussion on a topic which is inappropriate for the forum you can disregard this and I can delete this)
I have always found cyanuric acid a brilliant structure.. The tautomerism also applies to barbs. Not to mention cyanuric being essentially the compact perfect cyclic peptide haha.. The challenge is to switch from a super stable structure to an explosive or psychoactive one in the blink of an eye..
And also to form really sexy crystal lattices perhaps?
Last edited:
Why? I am curious about this. Could you elaborate your idea on why it should be that.Can I get E but in a hydroxy instead of the methoxy.. or with a methoxy on the adjacent carbon?
btw, D overlays nicely with 1-pentyl-3-(4'-methoxynapthoyl)indole and F although has 2 weird spiro, can be overlayed similary to ethyl-phenethylbarb
Are the methylene analogues of barbiturates active?

I believe that one or both of those deleted nitrogens would be needed to interact with a receptor, possibly via hydrogen bonding.
I would think by now medicinal chemists would have made all the possible variations on the barbituric acid nucleus & the different benzodiazepines as well. But you never know. Valium made Roche rich but they missed Xanax which made Upjohn rich.
Last edited:
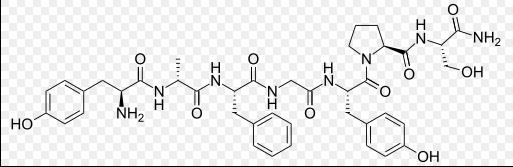
I've gotten interested in the potent opioid peptides found in the tropical poison dart frogs, particularly dermorphin which Wikipedia says has been used to dope race horses, so someone was or is making this synthetically, probably by Merrifield solid-phase. Wikipedia: Dermorphin is about 30–40 times more potent than morphine but theoretically may be less likely to produce drug tolerance and addiction (due to its high potency).[4] The amino acid sequence of heptapeptide dermorphin is H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2, so the only exotic component is the D-Alanine. See https://en.wikipedia.org/wiki/Solid-phase_synthesis. Interesting article on this peptide: https://www.paulickreport.com/news/ray-s-paddock/chasing-the-frog-keeping-up-with-slippery-cheaters/
Dermorphin is described in the literature as very potent & long-lasting, probably due to the D-Ala preventing its rapid enzymatic breakdown in the body. This is an IV drug but there are probably other routes of administration.
Hexapeptides are also active opioids. Activity is related to enzymatic stability (D-amino acid substitutions). One is H-Tyr-d-Ala-Phe-Gly-Tyr-Pro-OH, so the terminal serine in dermorphin can be eliminated.
I read some time ago about guys playing with fentanyl analogs. One guy would play while the other guy had Narcan (naloxone) handy if he should appear to OD. Because of its potency, correct dosing with this stuff would be tricky.
Dermorphin is described in the literature as very potent & long-lasting, probably due to the D-Ala preventing its rapid enzymatic breakdown in the body. This is an IV drug but there are probably other routes of administration.
Hexapeptides are also active opioids. Activity is related to enzymatic stability (D-amino acid substitutions). One is H-Tyr-d-Ala-Phe-Gly-Tyr-Pro-OH, so the terminal serine in dermorphin can be eliminated.
I read some time ago about guys playing with fentanyl analogs. One guy would play while the other guy had Narcan (naloxone) handy if he should appear to OD. Because of its potency, correct dosing with this stuff would be tricky.

Last edited:
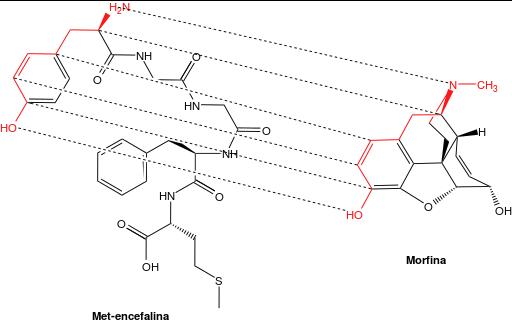
Tracing the binding correlations between dermorphin & morphine was interesting as it indicates that the main opioid pharmacophore is fixed on the first tyrosine residue of these opioid peptides. Those who want to dream up interesting molecules with potential biological activity might want to use tyrosine as a good starting point.
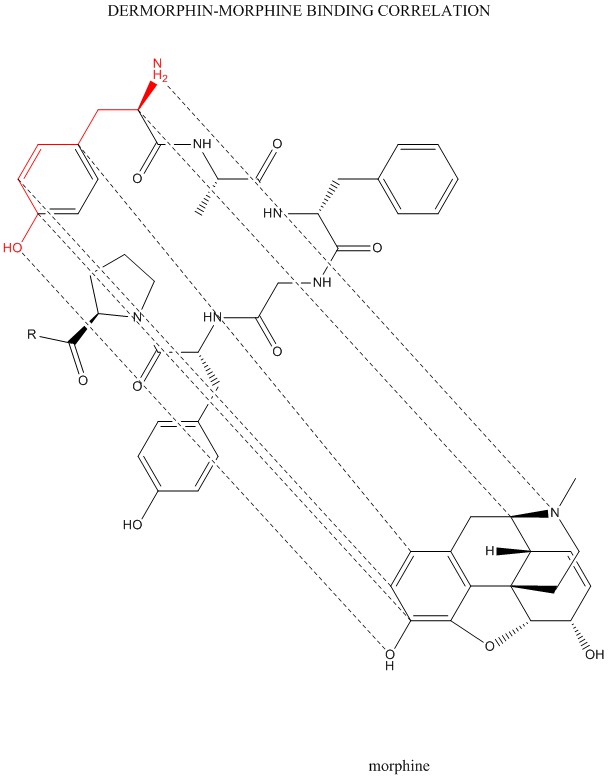
This graphic was generated using ChemDraw 17 Professional.

This graphic was generated using ChemDraw 17 Professional.
Last edited:
Yes it is. If you look at the amino acid sequences of all of your psychoactive peptides, they all start with tyrosine. It was interesting to find out why. It was also interesting to find out that these peptides can be a short as 4-5 residues, all starting with Tyr, like Tyr-Gly-Gly-Phe, etc. That puts them within range of a well-trained & well-equipped experimentalist, as most of these amino acids are sold in health food & vitamin stores.
You could design all kinds of potentially active molecules starting from tyrosine.
The big problem I see with the very potent ones like dermorphin is dosing & safety. One would need a very accurate balance capable of measuring at least tenths of a milligram.
You could design all kinds of potentially active molecules starting from tyrosine.
The big problem I see with the very potent ones like dermorphin is dosing & safety. One would need a very accurate balance capable of measuring at least tenths of a milligram.
Last edited:
An interesting difference between the dermorphin & morphine molecules is the amine function. In dermorphin, it is a primary amine. In morphine, it is a tertiary amine. The amine group in tyrosine would make another point on which to build structural variations. Since at least one of the N-H bonds is responsible for hydrogen bonding with the receptor, converting it to a secondary amine may work. The substituent involved would be a methyl or higher alkyl group.
Another interesting modification would be to esterify the phenol function in tyrosine which would make it more lipid soluble & better able to cross the blood-brain barrier. Or it could be converted into an ether.
Another interesting modification would be to esterify the phenol function in tyrosine which would make it more lipid soluble & better able to cross the blood-brain barrier. Or it could be converted into an ether.
Last edited:
The structural relationship between the active opioid alkaloid in kratom & morphine is not immediately apparent, but as shown in the attached graphic I can establish 6 points of correlation between mitragynine & morphine, the same number of correlation points I observed between dermorphin & morphine. It is not surprising that the FDA came to the same conclusion.
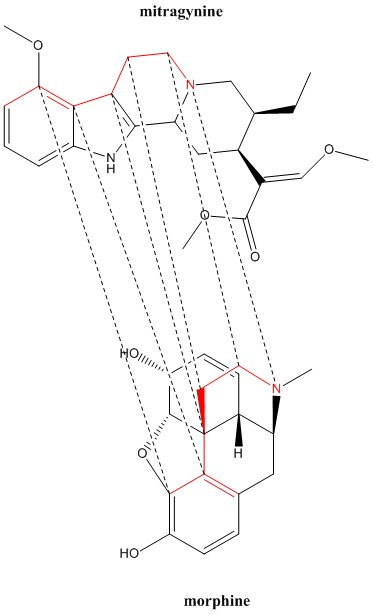
Graphic created using ChemDraw 17 Professional.

Graphic created using ChemDraw 17 Professional.
Last edited:
Here is the 6 point binding correlation for the opioid tramadol against morphine. The dotted line between the phenyl rings could not be drawn due to it being too close to another.
I don't see the usefulness of the hydroxyl group on the cyclohexane ring or the O-methyl group of tramadol. In fact, the major metabolite of tramadol is desmetramadol which lacks the O-methyl group & is a more potent opioid.
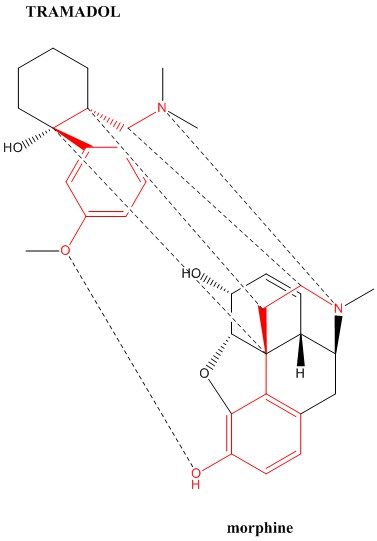
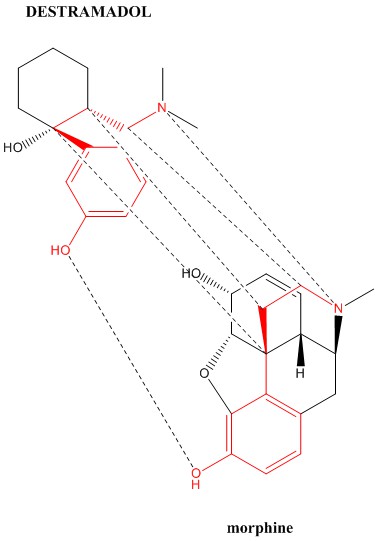
I don't see the usefulness of the hydroxyl group on the cyclohexane ring or the O-methyl group of tramadol. In fact, the major metabolite of tramadol is desmetramadol which lacks the O-methyl group & is a more potent opioid.


Last edited:
Tapentadol
This is a quick-acting, long-acting opoid that also inhibits the reuptake of norepinephrine. It is also an interesting molecule from the POV of having 2 chiral centers, which creates a mixture of diasteriomers than can be separated by recrystallization. The (R,R) isomer is the commercial product & also the weaker analgesic. The DEA listed it as Schedule II, in the same category as morphine & fentanyl. I assume that the other (S,S) diasteriomers are more active though the Grunenthal process patent makes only the (R,R) diasteriomer & makes no claims about its others (R,S, S,R, S,S). See https://www.sciencedirect.com/science/article/pii/S1110093113000239
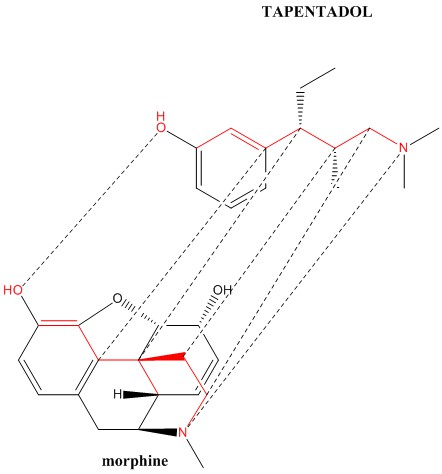
This is a quick-acting, long-acting opoid that also inhibits the reuptake of norepinephrine. It is also an interesting molecule from the POV of having 2 chiral centers, which creates a mixture of diasteriomers than can be separated by recrystallization. The (R,R) isomer is the commercial product & also the weaker analgesic. The DEA listed it as Schedule II, in the same category as morphine & fentanyl. I assume that the other (S,S) diasteriomers are more active though the Grunenthal process patent makes only the (R,R) diasteriomer & makes no claims about its others (R,S, S,R, S,S). See https://www.sciencedirect.com/science/article/pii/S1110093113000239

Last edited:
- Status
- Not open for further replies.