polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
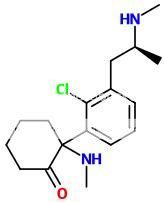
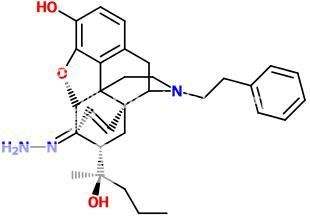
^ In fact, morphinelike compounds need to have the phenolic 3-hydroxy group or they lose potency completely... (thats why codeine or heroin isn't active before it's hydrolyzed in the body) You should connect that tropane part only to the cyclohexene ring.
EDIT: I was talking about the first molecule, where theres a carboxyl at 3-position.
EDIT: I was talking about the first molecule, where theres a carboxyl at 3-position.