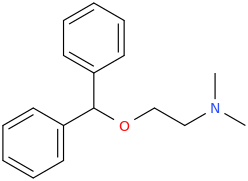
-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
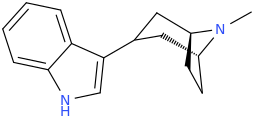
Ascaridole is notable, for not only being one of the only known natural organoperoxides, the other source being the Boldo plant. But for being, as with other organoperoxides, explosive. Not counting the likes of biological trioxidane generation as a reactive and short lived intermediate.
Re: above molecules
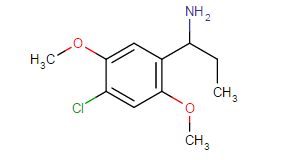
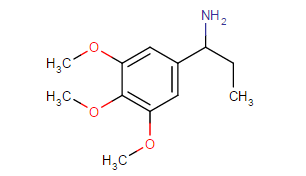
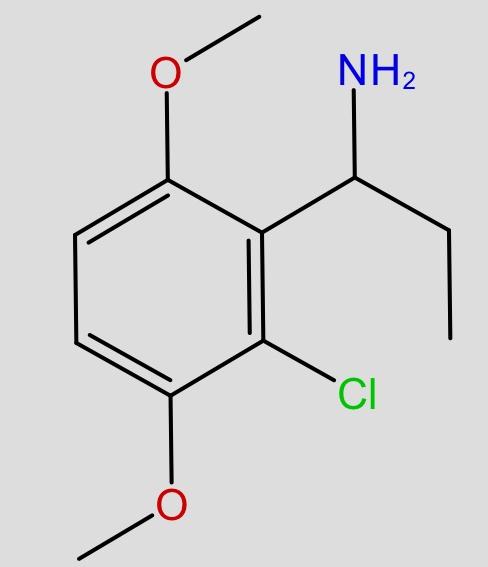
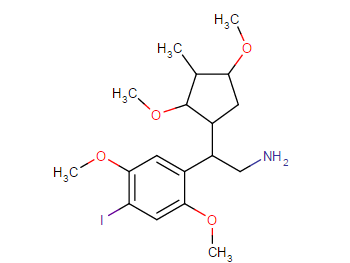
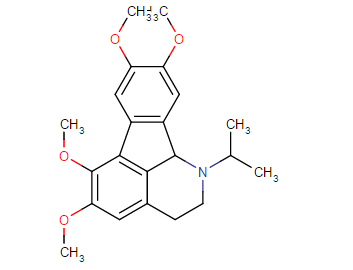
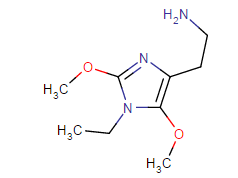
Those are hughly probable to be active, but may have very high tendency to cause headache (no high tho, just altered perception)
I have tried (+)- isomer and (-)- isomer of 2-amino-2-phenylethane before, its like the above structure without substitutons on that phenyl and replace that ethyl chain with methyl.
A lot of headache from like 20mg
Those are hughly probable to be active, but may have very high tendency to cause headache (no high tho, just altered perception)
I have tried (+)- isomer and (-)- isomer of 2-amino-2-phenylethane before, its like the above structure without substitutons on that phenyl and replace that ethyl chain with methyl.
A lot of headache from like 20mg
pr0d1gy
Bluelighter
- Joined
- May 1, 2009
- Messages
- 547
Re: above molecules
Those are hughly probable to be active, but may have very high tendency to cause headache (no high tho, just altered perception)
I have tried (+)- isomer and (-)- isomer of 2-amino-2-phenylethane before, its like the above structure without substitutons on that phenyl and replace that ethyl chain with methyl.
A lot of headache from like 20mg
Any hypothesis on the properties of these compounds with the carbon chain shortened by one, ie: the 2-amino-2-phenylmethanes
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
Any hypothesis on the properties of these compounds with the carbon chain shortened by one, ie: the 2-amino-2-phenylmethanes
That's not a thing, what you are describing is benzylamine... I don't believe compounds like that are 'neurotransmitter-like', that distance from the phenyl ring to the amine is probably quite critical.
Also, 2-amino-2-phenylethane is incorrect nomenclature in the first place
But that is not really the usual approach approach either, actually the 'order' of naming is 1-phenylethylamine (hey we know that name!), or alpha-phenethylamine as opposed to the beta-phenethylamines we have all come to know and love ;P
Last edited:
fentanyll and carba-analogs bound to the μ opioid receptor in the activated state.. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2848705/
looks interesting
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
Alpha-methylbenzylamine is an MAO(a)I IIRC. Pretty sure its been described as alkylating some other CNS constituent, although I can't remember what now.
- Status
- Not open for further replies.