-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
I Like to Draw Pictures of Random Molecules
- Thread starter nuke
- Start date
- Status
- Not open for further replies.
Bagseed
Bluelighter
- Joined
- Jul 22, 2010
- Messages
- 4,042
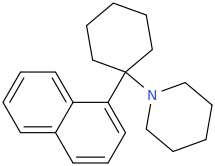
Naphcyclidine?
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
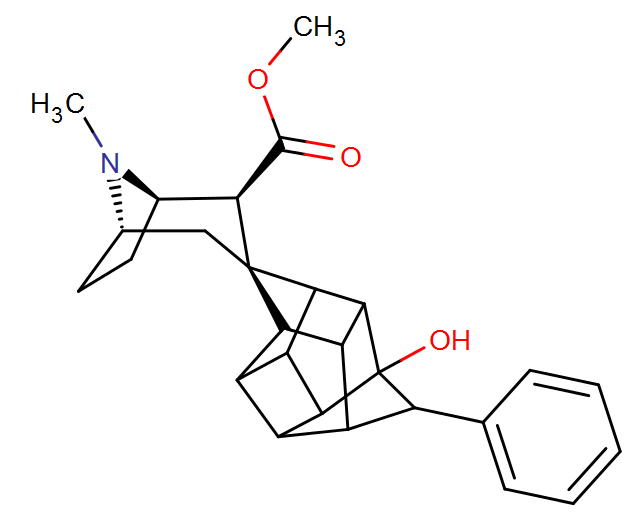
^Sigma agonist type inspired
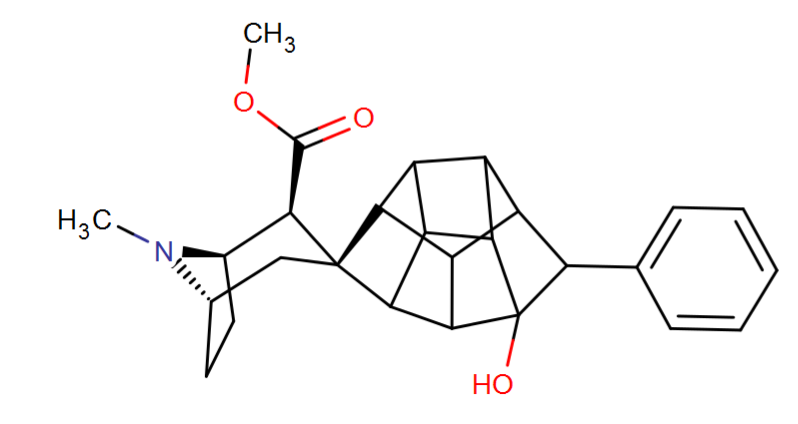
^Cleaned it doesn't look too much different in ultimate length than RTI-298 type and their "remote binding domain"
Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
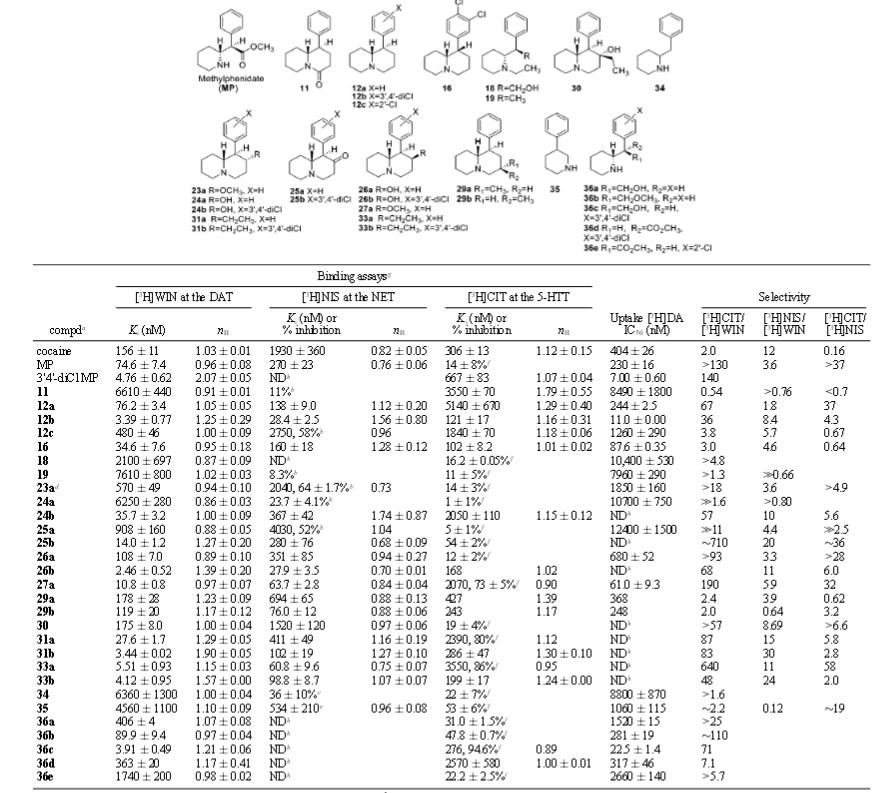
Some interesting restricted rotational analogs of methylphenidate. Since finding this paper I'm going to be harried with the urge to add these tables to my analogs list on WP, already got it printed off, they are interesting, going by the assumption that decreased conformational flexibility leads to increased binding:



aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
Some interesting restricted rotational analogs of methylphenidate. Since finding this paper I'm going to be harried with the urge to add these tables to my analogs list on WP, already got it printed off, they are interesting, going by the assumption that decreased conformational flexibility leads to increased binding:


Thanks for this find
Retired Trashcan
Bluelighter
- Joined
- Jan 29, 2014
- Messages
- 159
I'm new to this thread, but not to BL. I love looking at all of you guys and gals crazy molecules and discussion. Excellent fun for someone back in school taking chem classes. I can't draw yet, but I'd be curious to see some phenibut/GABA drugs tweaks so to speak. Thanks and keep up the great work! :D
EDIT: Damn that shaggyfin was a trip... lol
Someday I'll be educated enough to draw them, but til then, are question and comments welcome from a 'noob'?
EDIT: Damn that shaggyfin was a trip... lol
Someday I'll be educated enough to draw them, but til then, are question and comments welcome from a 'noob'?
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
I imagine so if it doesn't flood the thread, but you can always switch to PMs..  nice that you take an interest, hopefully you'll keep that up..
nice that you take an interest, hopefully you'll keep that up..
Here's an idea:
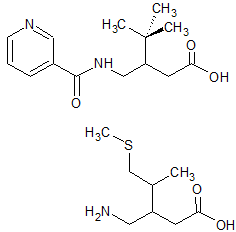
The above is the niacinoyl amide of some novel fantasy gabapentinoid... the idea is that as in picamilon you'd hope to get it across the BBB before hydrolyzing to the parent compounds whereas most gabapentinoids fail since they use the system L transporter. Pregabalin and 4-methylpregabalin are like mimics of the endogenous substrate for that transporter: leucine and isoleucine... if it doesn't look like those, the transporter is not interested. So would a niacinoyl facilitate this? By the way what transports phenibut across the BBB?
The original idea was to draw a niacinoyl on one of the gabapentinoids Pfizer designed (mentioned in Beliotti paper?) that were found to be quite effective in vitro but are not accepted by the amino acid transporter... however I have no access to the full paper so I don't know what actually effective analogues are - so I drew a t-Bu for the basic idea.
The one below is the methionine analogue of pregabalin but I have no clue if it has a chance in hell to use system A.
Picamilon is quite nice by the way, much less sedating than most other GABAergics or GABA itself. The above compounds are not GABAergics to of course.
Here's an idea:

The above is the niacinoyl amide of some novel fantasy gabapentinoid... the idea is that as in picamilon you'd hope to get it across the BBB before hydrolyzing to the parent compounds whereas most gabapentinoids fail since they use the system L transporter. Pregabalin and 4-methylpregabalin are like mimics of the endogenous substrate for that transporter: leucine and isoleucine... if it doesn't look like those, the transporter is not interested. So would a niacinoyl facilitate this? By the way what transports phenibut across the BBB?
The original idea was to draw a niacinoyl on one of the gabapentinoids Pfizer designed (mentioned in Beliotti paper?) that were found to be quite effective in vitro but are not accepted by the amino acid transporter... however I have no access to the full paper so I don't know what actually effective analogues are - so I drew a t-Bu for the basic idea.
The one below is the methionine analogue of pregabalin but I have no clue if it has a chance in hell to use system A.
Picamilon is quite nice by the way, much less sedating than most other GABAergics or GABA itself. The above compounds are not GABAergics to of course.
Retired Trashcan
Bluelighter
- Joined
- Jan 29, 2014
- Messages
- 159
Solipsis;13713494 Here's an idea: [IMG said:http://i.imgur.com/KwJdJHW.png[/IMG]
By the way what transports phenibut across the BBB?
The one below is the methionine analogue of pregabalin but I have no clue if it has a chance in hell to use system A.
Picamilon is quite nice by the way, much less sedating than most other GABAergics or GABA itself. The above compounds are not GABAergics to of course.
Thank you. I won't ask all the time. Just watching, learning, studying. Chemistry is the most FASINATING I know of. Phenibut crosses on the phenyl ring, pregabalin, btw (I know you know) crosses(Very Well at 90% BA Oral) on the isobutyl ring.
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
No I mean what is the transport mechanism, not what functional group makes it able to cross some way or another..  The BBB has various kinds like iirc passive diffusion or what's it called - just a sort of encapsulation of a compound that gobbles a molecule up and poops it out on the other side, and proteins designed to transport necessary chemicals into your brain like those amino acid transporter systems..
The BBB has various kinds like iirc passive diffusion or what's it called - just a sort of encapsulation of a compound that gobbles a molecule up and poops it out on the other side, and proteins designed to transport necessary chemicals into your brain like those amino acid transporter systems..
Phenibut just diffuses on account of its log P?
But you are correct about the groups substituted on GABA for those compounds..
Still enjoying previous pages?
Phenibut just diffuses on account of its log P?
But you are correct about the groups substituted on GABA for those compounds..
Still enjoying previous pages?
aced126
Bluelighter
- Joined
- May 18, 2015
- Messages
- 1,047
Thank you. I won't ask all the time. Just watching, learning, studying. Chemistry is the most FASINATING I know of. Phenibut crosses on the phenyl ring, pregabalin, btw (I know you know) crosses(Very Well at 90% BA Oral) on the isobutyl ring.
This.
Retired Trashcan
Bluelighter
- Joined
- Jan 29, 2014
- Messages
- 159
Yeah its really the 2C-B's knees!
Still enjoying previous pages?
I think I've made it to page 112, all the way from page one. My mind is blown that there is all this knowledge out there to be absorbed on this page. Y'all come up with ideas, critique each others work, crack jokes. Best page on BL (Gee, sorry Lounge

EDIT: I believe LAT-1 is the transporter Solipsis. Damn, I feel like I'm in class, jk.
Last edited:
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
Yeah makes me wish I didnt eventually flunk it all in college..
People here do propose crazy chemicals, after all it is the random molecules thread so anything will do... but I guess there is different kinds of crazy, and at some point some kinds are imo a little pointless - others are entertaining, interesting etc, or educating to the poster or just in general.. imo - Mr fin didn't get that message, but don't wait too long participating either.. but yeah some fundamentals of organic chemistry and checking out multiple compounds in a 'family' or 'category' might be a minimum..
Why do you believe its the LAT-1 (I think this refers to the system L i was previously talking about ^ )? It's fine if you don't post sources from digging around online - i do it all the time, if you instead provide a rationale :D again imo
People here do propose crazy chemicals, after all it is the random molecules thread so anything will do... but I guess there is different kinds of crazy, and at some point some kinds are imo a little pointless - others are entertaining, interesting etc, or educating to the poster or just in general.. imo - Mr fin didn't get that message, but don't wait too long participating either.. but yeah some fundamentals of organic chemistry and checking out multiple compounds in a 'family' or 'category' might be a minimum..
Why do you believe its the LAT-1 (I think this refers to the system L i was previously talking about ^ )? It's fine if you don't post sources from digging around online - i do it all the time, if you instead provide a rationale :D again imo
Retired Trashcan
Bluelighter
- Joined
- Jan 29, 2014
- Messages
- 159
Why do you believe its the LAT-1 (I think this refers to the system L i was previously talking about ^ )? It's fine if you don't post sources from digging around online - i do it all the time, if you instead provide a rationale :D again imo
I was sure when I was reading up on PBT that I saw that LAT was the transporter, but I'm a rookie in that department for sure.

sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
Some interesting restricted rotational analogs of methylphenidate. Since finding this paper I'm going to be harried with the urge to add these tables to my analogs list on WP, already got it printed off, they are interesting, going by the assumption that decreased conformational flexibility leads to increased binding:
Hm, and some of these remind me of the "seco" opioids / restricted rotation pethidine anaogs.
Cmpd 12a looks particularly tasty
H ---> NO2 increase mu ~2000x (like in https://en.wikipedia.org/wiki/Etonitazene
2H ---> 2NO2 increase mu ~ 4,000,000 times (theoretical speculation!
Fenta is ~ 100xmorphine therefore
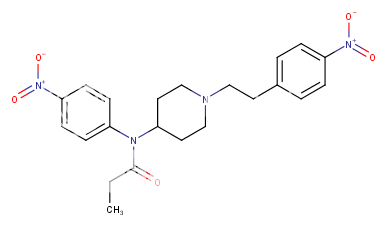
about 400,000,000X more potent than morphine as a mu opioid agonist!?
or more reasonable:
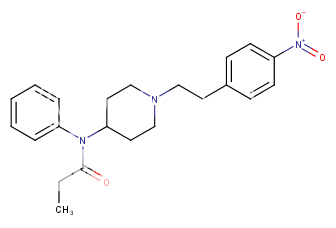
Only 200,000x morphine! ..
..
2H ---> 2NO2 increase mu ~ 4,000,000 times (theoretical speculation!
Fenta is ~ 100xmorphine therefore

about 400,000,000X more potent than morphine as a mu opioid agonist!?

or more reasonable:

Only 200,000x morphine!
Last edited:
- Status
- Not open for further replies.