punkftl
Bluelighter
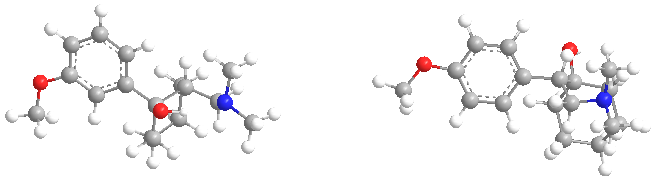
according to Wiki...Effexor has a similar chemical structure to the opioid derivative Tramadol. it acts as an agonist at the mu-opioid receptor.
could this explain why Effexor has some of the worst withdrawals of any antidepressant? usually antidepressants with the shortest half-life have the worst symptoms, but even Effexor XR, with it's longer half-life compared to immediate release Effexor, have almost the same severity.
AND Effexor is more effective in treating depression in heroin and other opioid dependent individuals. even though i am on 400mg. Wellbutrin SR. daily along with Methadone and that seems to work well also (combined with L-Tyrosine,
5-HTP, high doses DHA fish oil)
from Wiki
Venlafaxine (Effexor, Efexor) is an arylalkanolamine serotonin-norepinephrine reuptake inhibitor (SNRI)[2]. It has a similar chemical structure to the opioid derivative tramadol, and has the tertiary amine functional group necessary for µ-opioid receptor recognition (cf. lefetamine), and indeed, Venlafaxine acts as an agonist at the mu-opioid receptor. [3] [4]It is surprisingly effective in treating depression in heroin and other opioid addicts compared to all other conventional antidepressants.[5].
and a study:
Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects--a possible opioid involvement in severe depression?
Schreiber S, Bleich A, Pick CG.
Department of Psychiatry, Tel Aviv Sourasky Medical Center, Tel-Aviv University Sackler School of Medicine, Israel.
Abstract
The efficacy of each antidepressant available has been found equal to that of amitriptyline in double-blind studies as far as mild to moderate depression is involved. However, it seems that some antidepressants are more effective than others in the treatment of severe types of depression (i.e., delusional depression and refractory depression). Following studies regarding the antinociceptive mechanisms of various antidepressants, we speculate that the involvement of the opioid system in the antidepressants' mechanism of action may be necessary, in order to prove effective in the treatment of severe depression. Among the antidepressants of the newer generations, that involvement occurs only with venlafaxine (a presynaptic drug which blocks the synaptosomal uptake of noradrenaline and serotonin and, to a lesser degree, of dopamine) and with mirtazapine (a postsynaptic drug which enhances noradrenergic and 5-HT1A-mediated serotonergic neurotransmission via antagonism of central alpha-auto- and hetero-adrenoreceptors). When mice were tested with a hotplate analgesia meter, both venlafaxine and mirtazapine induced a dose-dependent, naloxone-reversible antinociceptive effect following ip administration. Summing up the various interactions of venlafaxine and mirtazapine with opioid, noradrenergic and serotonergic agonists and antagonists, we found that the antinociceptive effect of venlafaxine is influenced by opioid receptor subtypes (mu-, kappa1- kappa3- and delta-opioid receptor subtypes) combined with the alpha2-adrenergic receptor, whereas the antinociceptive effect of mirtazapine mainly involves mu- and kappa3-opioid mechanisms. This opioid profile of the two drugs may be one of the explanations to their efficacy in severe depression, unlike the SSRIs and other antidepressants which lack opioid activity.
could this explain why Effexor has some of the worst withdrawals of any antidepressant? usually antidepressants with the shortest half-life have the worst symptoms, but even Effexor XR, with it's longer half-life compared to immediate release Effexor, have almost the same severity.
AND Effexor is more effective in treating depression in heroin and other opioid dependent individuals. even though i am on 400mg. Wellbutrin SR. daily along with Methadone and that seems to work well also (combined with L-Tyrosine,
5-HTP, high doses DHA fish oil)
from Wiki
Venlafaxine (Effexor, Efexor) is an arylalkanolamine serotonin-norepinephrine reuptake inhibitor (SNRI)[2]. It has a similar chemical structure to the opioid derivative tramadol, and has the tertiary amine functional group necessary for µ-opioid receptor recognition (cf. lefetamine), and indeed, Venlafaxine acts as an agonist at the mu-opioid receptor. [3] [4]It is surprisingly effective in treating depression in heroin and other opioid addicts compared to all other conventional antidepressants.[5].
and a study:
Venlafaxine and mirtazapine: different mechanisms of antidepressant action, common opioid-mediated antinociceptive effects--a possible opioid involvement in severe depression?
Schreiber S, Bleich A, Pick CG.
Department of Psychiatry, Tel Aviv Sourasky Medical Center, Tel-Aviv University Sackler School of Medicine, Israel.
Abstract
The efficacy of each antidepressant available has been found equal to that of amitriptyline in double-blind studies as far as mild to moderate depression is involved. However, it seems that some antidepressants are more effective than others in the treatment of severe types of depression (i.e., delusional depression and refractory depression). Following studies regarding the antinociceptive mechanisms of various antidepressants, we speculate that the involvement of the opioid system in the antidepressants' mechanism of action may be necessary, in order to prove effective in the treatment of severe depression. Among the antidepressants of the newer generations, that involvement occurs only with venlafaxine (a presynaptic drug which blocks the synaptosomal uptake of noradrenaline and serotonin and, to a lesser degree, of dopamine) and with mirtazapine (a postsynaptic drug which enhances noradrenergic and 5-HT1A-mediated serotonergic neurotransmission via antagonism of central alpha-auto- and hetero-adrenoreceptors). When mice were tested with a hotplate analgesia meter, both venlafaxine and mirtazapine induced a dose-dependent, naloxone-reversible antinociceptive effect following ip administration. Summing up the various interactions of venlafaxine and mirtazapine with opioid, noradrenergic and serotonergic agonists and antagonists, we found that the antinociceptive effect of venlafaxine is influenced by opioid receptor subtypes (mu-, kappa1- kappa3- and delta-opioid receptor subtypes) combined with the alpha2-adrenergic receptor, whereas the antinociceptive effect of mirtazapine mainly involves mu- and kappa3-opioid mechanisms. This opioid profile of the two drugs may be one of the explanations to their efficacy in severe depression, unlike the SSRIs and other antidepressants which lack opioid activity.