They aren't gases. Liquids, mostly, although one of them, A242, IIRC, is a crystalline solid. There may be other solids, but from what I've read from experts (no, not mirzayanov) A-242 (novichok-9) is a solid. Nasty little buggers, some 5-10x as potent as VX, and apparently, and quite expectedly on my part, I'd guessed that they would, unlike other nerve agents, because of that dihalogenated formaldoxime group, be able to physically chew through skin and other tissue, in addition to causing systemic toxicity. If you look at that part of the structure, that substructure is basically phosgene oxime, or an analog of it, depending on which halogens are used (phosgene itself is carbonyl dichloride, (Cl2(C)=O and its a highly toxic gas, itself, classed with the pulmonary agents, whilst phosgene oxime is a solid, albeit a volatile one, that is classed as a 'nettle agent' due to its causing immediate pain upon contact of the solid or its vapor with skin, causing blanching, severe pain, itching, and after a while, a blackened scab forms and eventually is shed by the body. Corrosive properties pretty much)
And I've also read that having the oxime functional group already present in the novichok agents, prevents the Hagedorn oximes usually administered alongside atropine from binding and as they do normal nerve agents, dephosphorylating the serine residue in a critical site within the acetylcholinesterase enzyme structure which attacked and phosporylated by the nerve agents.
These oxime compounds USUALLY, with most nerve agents basically transfer that phosphoryl group to themselves, reactivating poisoned cholinesterase whilst the oxime-nerve agent complex is excreted. (Although unfortunately for anyone poisoned, they also have to get the oxime within a limited time period, which varies from agent to agent; and is not correlated to the lethal dosage of the agent, for example, VX can take up to 48 hours before the VX-cholinesterase complex 'ages' as the process is known, and a rearrangement takes place which renders the oxime reactivators useless, whilst if its soman that you get poisoned by, although it takes a greater weight of soman to kill you compared to VX, soman ages incredibly rapidly once it has inactivated acetylcholinesterase. If you don't get the oxime within ten minutes, the soman-cholinesterase complex has aged and you are, in a word, fucked; supportive treatment such as atropine only, due to the aging rendering the poisoned cholinesterase enzyme impervious to dephosphorylation with the oxime drug used.)
https://www.rferl.org/a/novichok-cr...al-poison-will-remain-a-mystery/29182827.html
Looks like my hunch from a few years back about these nerve agents, assuming one goes with the formulas which have the asymmetrical dihaloformaldoxime substructure rather than a tertiary amine in the same place, which given that behaviour I am inclined to believe, act as both nerve- and nettle-agents, able to chew through the skin even in solid form to deliver themselves into the open wound.
Was it sciencemadness.org that the novichok syntheses were posted on? wouldn't surprise me, IIRC members there have worked out syntheses for VX other than the classic route via agent QL.
And having a pesticide precursor+acetonitrile (methyl cyanide) be one route to novichok agents, that shouts 'binary weapon potential' there, especially if said pesticide precursor is liquid or dissolved in solvent for easy dispersal, acetonitrile is also a mobile, non-viscous liquid, so the two would mix together easily if made into a binary munition. Whereas for binary VX, IIRC the non QL portion, is solid elemental sulfur. Much less efficient to mix sulfur powder and a liquid, than two liquids.
Acetonitrile is about as viscous as say, ether, or acetone, lower alcohols like the one we drink, etc. I have some in the fridge as it happens, got about a liter of the stuff I use as a lab solvent (no, it isn't the fridge I keep my food in, I have a separate chemicals fridge. And no, I have absolutely not the least intention of synthesizing any of the novichok agents.)
Even if I did want to, I'd be too unhappy with the idea of it backfiring on me, save for the idea of a binary agent. But no, not my area of chemistry, at least, not on the practical side of things; not to say I don't take an interest on the academic side, but I've no wish to actually MAKE the things.
(I'm of the school of thought that goes 'its better to know what makes for a danger, or something that is a close analog of such a danger than not to know, hobby chemists SHOULD know what makes for say, a nitrogen or sulfur mustard, a nerve agent, etc. because then when it comes to designing a synthetic route to something, you can then spot a particular compound or intermediate that is, or would give rise to a CW agent. I've been served well by knowing HOW and WHY as well as WHAT makes for such nasty chemical warfare agents. For example, I've been wanting some Hunig's base, diisopropylethylamine, a sterically hindered, non-nucleophilic strong organic amine base, and i have diisopropylaminoethanOL.
Problem is, a halogen beta to an amine group, especially if its a bis-compound of some sort, makes for a good candidate for behaviour as a nitrogen mustard. So halogenating the alcohol and using it as a leaving group was discarded as an idea. Now going to use a tosyl group as the leaving group of my choice. Why? and why am I choosing to proceed?
Because of knowledge about how nitrogen mustards (along with sulfur mustards, the nitrogen mustards are blister agents, although also in the case of the nitrogen mustards, and variants of them, used for treating leukaemias) work.
First, they all have a bis- or tris-haloalkyl group, on an amine in nitrogen mustards, as a bis-(2-haloalkyl)alkylthioether in sulfur mustards, and a conventional R-O-R ether in oxygen mustard and a couple others. The two reactive leaving groups (the halogens) act to bridge across the two sugar-phosphodiester backbone strands of DNA, one leaving group, in the second stage of the attack, bonding one side of the mustard agent to one strand and the other, to the other, forming a crosslink.
The first step of attack, is formation of an aziridinium ion, which alkylates DNA, attacking at guanine, Then the aziridinium, a highly reactive intermediate, reacts and crosslinks the DNA strands, and during replication of genetic material, we have enzymes that 'proof-read' the genetic code prior to translation, and its basically like sticking superglue in a lock, the glue jams up the lock, while the nitrogen mustard jams the DNA strand with its crosslinkages, causing the proofreading enzymes to be unable to get past the blockage, and as a result, it registers as a damaged section of genetic material, and the cell is then forced to undergo apoptosis (programmed cell death), and it dies.
Whilst the aziridinium cation and its alkylation of DNA itself doesn't cause the horrific blistering effect of the vesicant chemical weapons class, thats the crosslinking in action, the aziridinium ion is requisite for this to happen. It so happens that in the case of diisopropylethylamine (Hunig's base, what I'm after), the two big, bulky isopropyl groups one either side immediately adjacent to the ethyl group (or ethanol group in my case, I need to remove the alcohol)
These big bulky buggers allow for a proton to bind, but not larger groups, so it can act as a base, but itself won't end up acylated, alkylated or whatever else, it just allows for smooth, clean deprotonations, and also, tosylate, whilst an excellent leaving group, better than iodide, which I would have used, the iodide might be volatile to some degree, whilst the tosylate is almost certainly a solid. And sterically speaking there should be no room for the aziridinium cation to form, in the case of diisopropylaminoethanol tosylate. So solid, nonvolatile, safer to handle. Only one, not two reactive leaving groups (the tosyl group), giving this intermediate no way to form those nasty crosslinkages between DNA strands IF it can even cyclize initially to form the aziridinium, which due to the aforementioned steric constraints, I'm pretty confident it cannot, and as such, atypically, due to its specific structure, diisopropylaminoethyl tosylate should not be capable of the virulent cytotoxicity of the mustards, despite having a great leaving group beta to the amine.
See? knowledge works both ways, for evil and for good. Knowing how the mustard 'gases' (they are liquids actually) work, means I spotted the potential danger, and could then analyse it in greater detail, work the problem and the risks out. And in this case, find them to be negligible and such as will be safe to proceed with and prepare my Hunig's base. Do I WANT to make mustard 'gas' blister weapons? do I hades! but BECAUSE I know exactly how they ARE made, I know how NOT to make them by accident, and know what characteristics of a molecule will make it a probable mustard-type blister agent, or a nerve agent etc. etc.




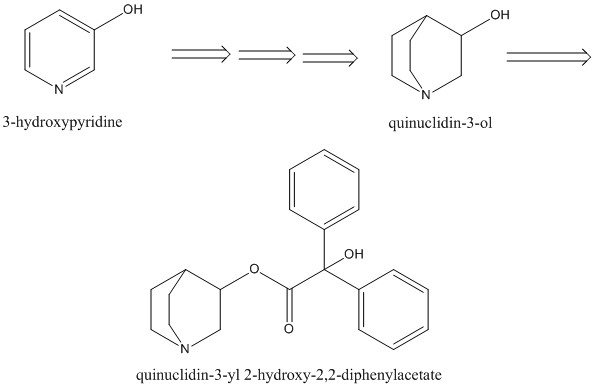
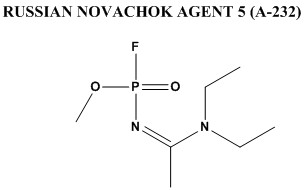
 The other precursor is going to be pretty virulent in its own right, that dihalogenated formaldoxime, its phosgene oxime with the halogens tweaked, could doubtless be made via reduction of chloropicrin analogs, at least thats where I'd start, if I were to have the mind to cook up anything quite so nasty, and even chloropicrin is pretty nasty shit. I wouldn't relish working with it as an intermediate for anything much, and I'd bet fluorinated analogs of it would be even more unpleasant, Think something along the lines of 'tear gas on steroids>phosgene oxime analog (phosgene oxime is classified as a nettle agent, somewhat similar in effect to sulfur/nitrogen mustard blister agents, and has a high vapor pressure, producing toxic effects within minutes of exposure, downright nasty stuff)>novichok type agent', so thats a pretty unpleasant set of procedures, that hopefully at least, would weed out plenty of inept allah-basher types before they ever got off the starting block, assuming they don't succeed and have their end product do the job.
The other precursor is going to be pretty virulent in its own right, that dihalogenated formaldoxime, its phosgene oxime with the halogens tweaked, could doubtless be made via reduction of chloropicrin analogs, at least thats where I'd start, if I were to have the mind to cook up anything quite so nasty, and even chloropicrin is pretty nasty shit. I wouldn't relish working with it as an intermediate for anything much, and I'd bet fluorinated analogs of it would be even more unpleasant, Think something along the lines of 'tear gas on steroids>phosgene oxime analog (phosgene oxime is classified as a nettle agent, somewhat similar in effect to sulfur/nitrogen mustard blister agents, and has a high vapor pressure, producing toxic effects within minutes of exposure, downright nasty stuff)>novichok type agent', so thats a pretty unpleasant set of procedures, that hopefully at least, would weed out plenty of inept allah-basher types before they ever got off the starting block, assuming they don't succeed and have their end product do the job.