Ham-milton
Bluelighter
- Joined
- Jul 20, 2007
- Messages
- 5,746
Hey, I know that Nichols has looked at some of these sorts of things, especially with MDMA, and didn't find them to be as active, but has there been any research into how the pure stimulant analogues are?
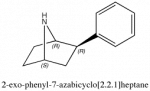
I'm thinking of something like this analogue of phenmetrazine

As I recall, these sorts of analogues had less peripheral effects, but maintained activity although there was a loss of potency. Does that sound accurate?
I'm thinking of something like this analogue of phenmetrazine

As I recall, these sorts of analogues had less peripheral effects, but maintained activity although there was a loss of potency. Does that sound accurate?
On a side note, does anyone know if bemegride or it's relatives had potential as recreational drugs? I don't think it has any analogues that are stimulants, though. It's described in one of the Future Synthetic Drugs of Abuse as having "obvious" abuse potential. I can't find though how it works. It a gaba-antagonist or is a dopaminergic like other stimulants?