Jaw Clenching
Bluelighter
- Joined
- Feb 4, 2005
- Messages
- 551
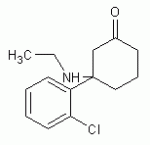
3-(2-chlorophenyl)-3-(ethylamino)cyclohexanone
3-(2-chlorophenyl)-3-(ethylamino)cyclohexanone
Ketamine analog - I've been told it has very similar effects as Ketamine and is about 4 times as potent by weight.
- Ketamine is 3-(2-chlorophenyl)-3-(methylamino)cyclohexanone -
3-(2-chlorophenyl)-3-(ethylamino)cyclohexanone
Ketamine analog - I've been told it has very similar effects as Ketamine and is about 4 times as potent by weight.
- Ketamine is 3-(2-chlorophenyl)-3-(methylamino)cyclohexanone -