Mr Blonde
Bluelighter
- Joined
- Oct 1, 2006
- Messages
- 13,813
OK, so from what I've read, in order for molecules to pass the blood-brain-barrier they have to have lipid solubility. Lipids are fat-soluble molecules, right?
Secondly, the blood-brain-barrier makes it difficult for hydrophillic molecules to pass through as well.
So...
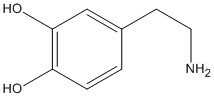
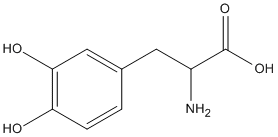
1) What is the difference between, for example, L-DOPA and dopamine that makes one lipid soluble and the other not? L-DOPA has a carboxylic acid group added...that makes a molecule lipid soluble? In all cases or just some?
Dopamine
L-DOPA
2) It's difficult for hydrophillic molecules to cross the BBB...but many recreational drugs are hydrophillic. Does lipid solubility win rock-paper-scissors over the obstacles facing hydrophillic molecules?
I'd appreciate some responses, I'm a novice at this neuropharmacology stuff and I'm always trying to learn some more...would like to see the ADD neuropharmocology text get finished some day!
Secondly, the blood-brain-barrier makes it difficult for hydrophillic molecules to pass through as well.
So...
1) What is the difference between, for example, L-DOPA and dopamine that makes one lipid soluble and the other not? L-DOPA has a carboxylic acid group added...that makes a molecule lipid soluble? In all cases or just some?
Dopamine

L-DOPA

2) It's difficult for hydrophillic molecules to cross the BBB...but many recreational drugs are hydrophillic. Does lipid solubility win rock-paper-scissors over the obstacles facing hydrophillic molecules?
I'd appreciate some responses, I'm a novice at this neuropharmacology stuff and I'm always trying to learn some more...would like to see the ADD neuropharmocology text get finished some day!
