Nagelfar
Bluelight Crew
- Joined
- Nov 23, 2007
- Messages
- 2,527
Way more complex than "closed to out" or "open to out" conformation on DAT binding...
Novel C-1 Substituted Cocaine Analogs Unlike Cocaine
or Benztropine
apparently, they have been able to render C1 position analogues of cocaine that bind in an open-to-out fashion, but are non-stimulating like the closed-to-out kind. I edited WP with thus:
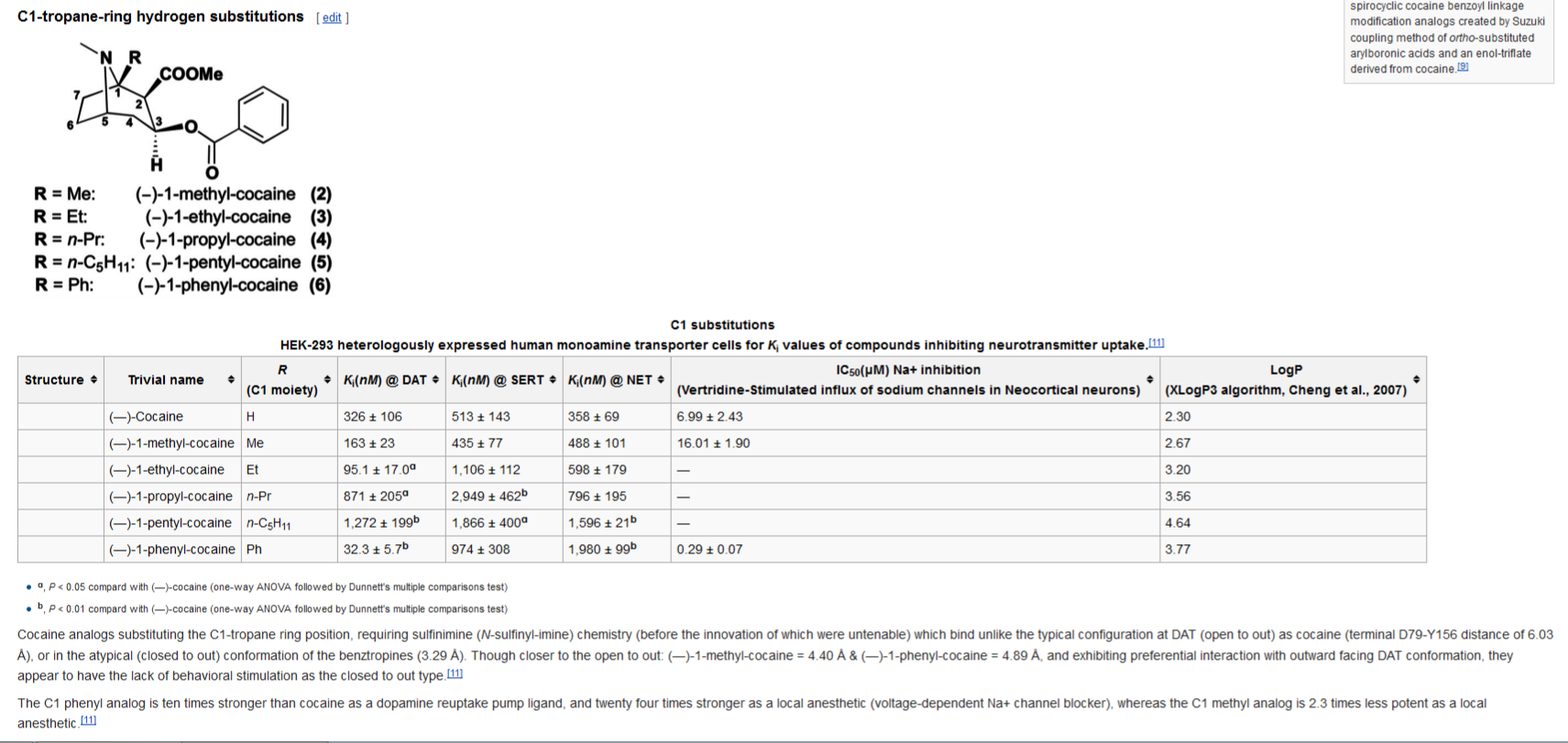
A=cocaine, B=C1-methyl-cocaine, C=C1-phenyl-cocaine & D=benztropine
(I really want to put an acetoxy group at C1!!!!! )
)
Novel C-1 Substituted Cocaine Analogs Unlike Cocaine
or Benztropine
apparently, they have been able to render C1 position analogues of cocaine that bind in an open-to-out fashion, but are non-stimulating like the closed-to-out kind. I edited WP with thus:

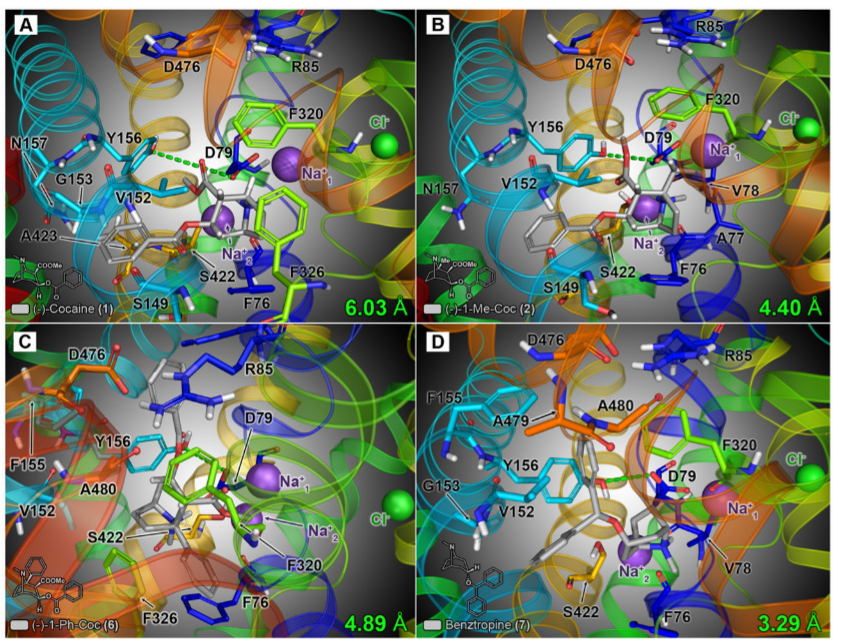
Final energy-minimized poses of the model hDAT/ligand complexes are shown in Fig. 8. After flexible docking, cocaine was oriented such that the protonated tropane amine faced the S1 site residues of TM1 and exhibited strong molecular interactions with residues Asp79 (via a hydrogen bond) and Phe76 (via a cation-pi interaction with the aromatic side chain of Phe76).
The 3-beta-benzoyloxy and 2-beta-carbomethoxy moieties were positioned toward TMs 3 and 8, enveloped by the side chains of residues Val152, Gly153, Tyr156, and Asn157, as well as Ser422 and Ala423 (Fig. 8A). The docking orientation of cocaine and the binding-pocket residues found were to be consistent with those reported by Beuming et al. (2008) in their docking model of cocaine at the DAT S1 site.
The docking model of 2 (1-methyl-cocaine) suggested that the analog binds in a similar manner as cocaine, with the extra methyl group oriented toward Asp79 and surrounding residues of TM1 (Ph76, Ala77, and Val78), but still readily accommodated within the binding pocket (Fig. 8B).
In contrast, the additional steric heft of the C-1 phenyl substituent of 6 (1-phenyl-cocaine) was not as readily accepted, and the compound adopted an entirely different binding orientation than cocaine and 2 (Fig. 8c). The extra C-1 phenyl group was positioned downward (toward the cytosolic vestibule), adjacent to the aromatic side chains of residues Phe76 and Phe326. In addition, the 3-beta-benzoyloxy moiety was oriented upward, slightly above the extra-cellular vestibule gating network residues Arg85, Phe320, and Asp476, enabling formation of a cation-pi interaction between the benzoyloxy aromic ring and Arg85

A=cocaine, B=C1-methyl-cocaine, C=C1-phenyl-cocaine & D=benztropine
(I really want to put an acetoxy group at C1!!!!!
