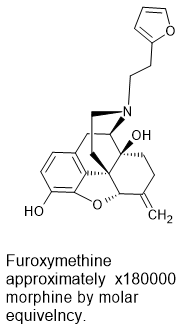
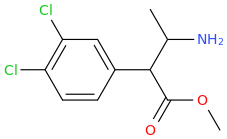
polymath
Bluelight Crew
- Joined
- Nov 4, 2010
- Messages
- 1,884
In another thread, I mentioned the possibility that the doubling of brain kynurenic acid levels by high doses of L-tryptophan (5 g/day) could possibly potentiate the effect of dissociatives, as kynurenate blocks the glycine binding site on NMDA receptors. Looking at some publications, it also seems that COX-1 inhibitors such as aspirin and paracetamol (acetaminophen) cause a similar increase in brain kynurenic acid concentration. In this article, this has been tested in rats with indomethacin. NMDAr glycine site blockers are also known to cause an anxiolytic effect, and this is also seen with paracetamol and aspirin, which makes me suspect that this effect of NSAIDs could be caused by them affecting metabolic kynurenate production. The anti-anxiety effect of paracetamol is usually attributed to its influence on endocannabinoid metabolism, but the kynurenic acid pathway could also be a factor in this. The possibility of aspirin or paracetamol potentiating the effect of ketamine, PCP and other dissociatives is somewhat significant, because someone could easily take that combination without knowing that there can be a synergistic effect.
Curiously, the non-steroidal anti-inflammatory drug indomethacin has also caused psychotic symptoms to some people, as have some other drugs of that class as well, and it wouldn't be surprising if this is caused by it affecting the NMDA receptor.
Edit: looks like there is this thread on BL about HA-966, which is an NMDA glycine site blocker or partial agonist.
Curiously, the non-steroidal anti-inflammatory drug indomethacin has also caused psychotic symptoms to some people, as have some other drugs of that class as well, and it wouldn't be surprising if this is caused by it affecting the NMDA receptor.
Edit: looks like there is this thread on BL about HA-966, which is an NMDA glycine site blocker or partial agonist.
Last edited: