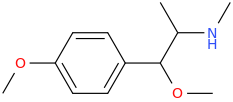
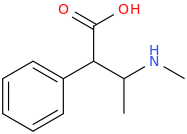
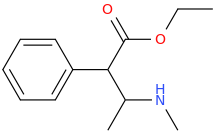
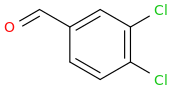
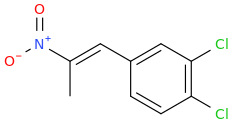
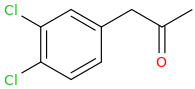
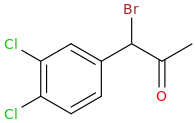
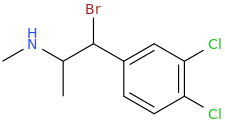
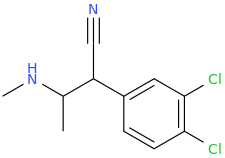
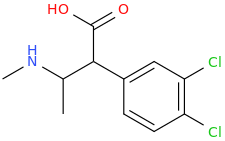
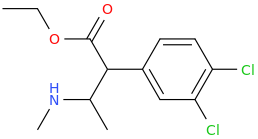
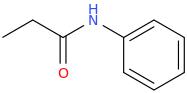
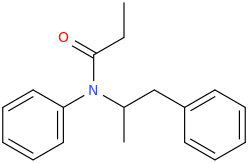
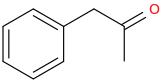
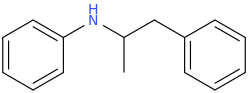
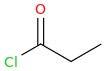
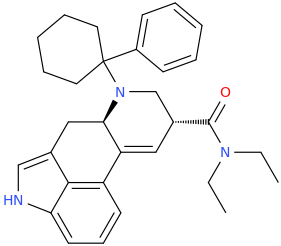
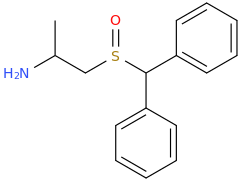
-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Rectify's molecular poetry thread
- Thread starter Dresden
- Start date
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,457
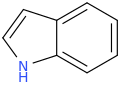
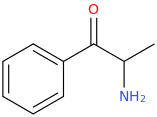
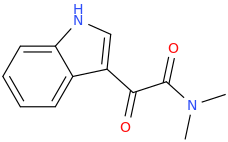
INDOLE
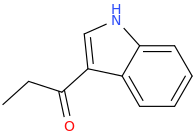
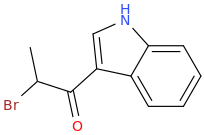
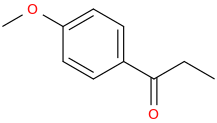
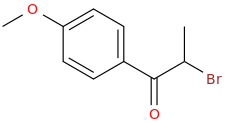
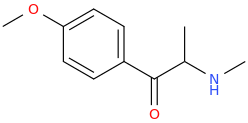
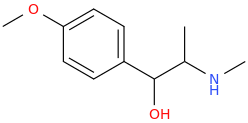
Protect carbonyl with ethylene glycol (antifreeze) and OH- to yield a spiro 1,3-dioxacyclopently functional group, then swap R-Br for R-NHCH3 and then finally deprotect with H+. The protecting group may only be necessary for certain primary R-NH2 containing compounds. I really don't know about that for certain.
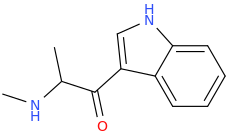
DAVE'S_KILLER_BREAD
3-(1-oxo-2-methylaminopropyl)-indole
Some Of The Best Bread Around!
Last edited:
- Joined
- May 11, 2011
- Messages
- 3,378
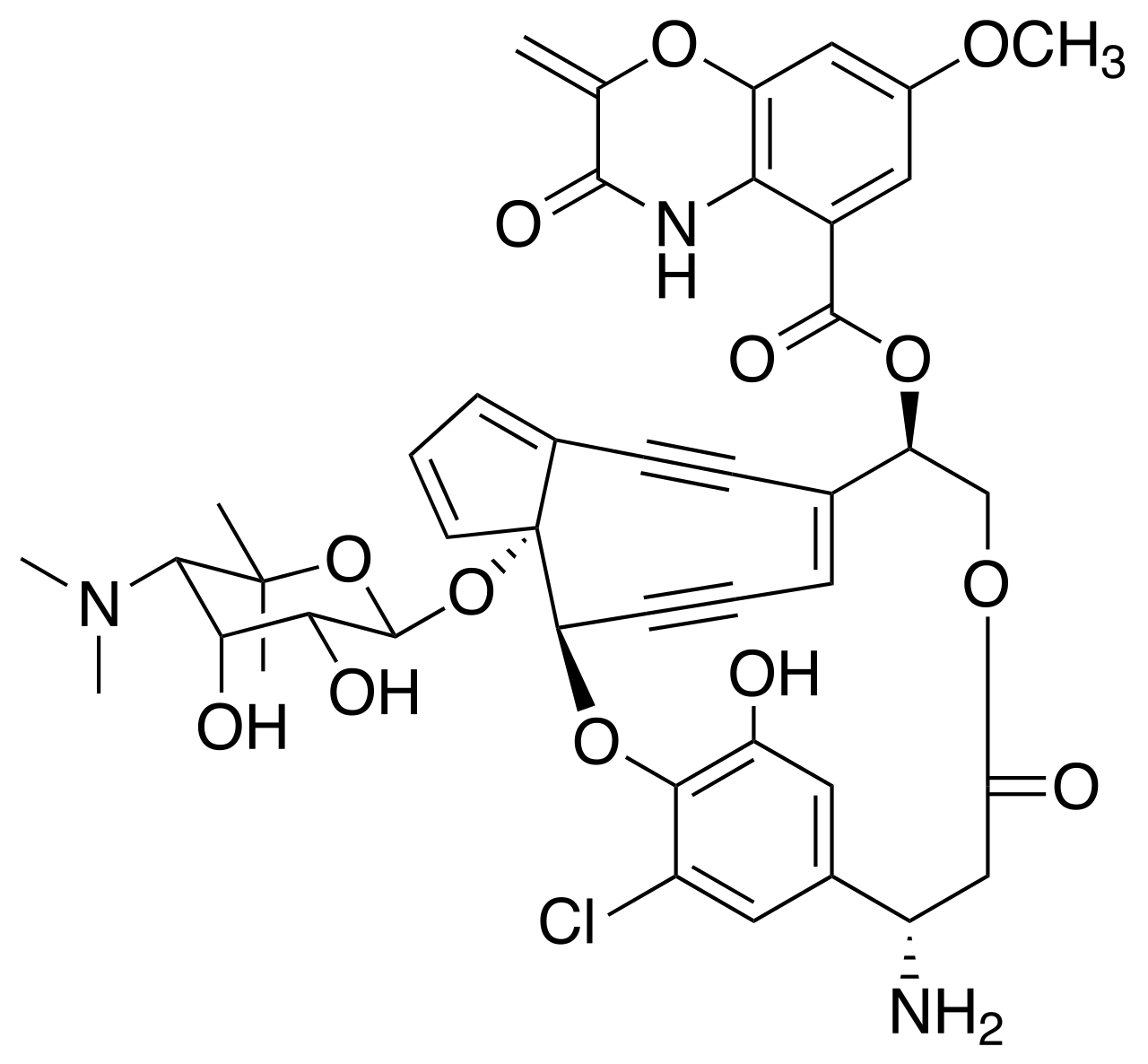
This isn't anything I came up with. It is a chemotherapeutic drug called lidamycin.
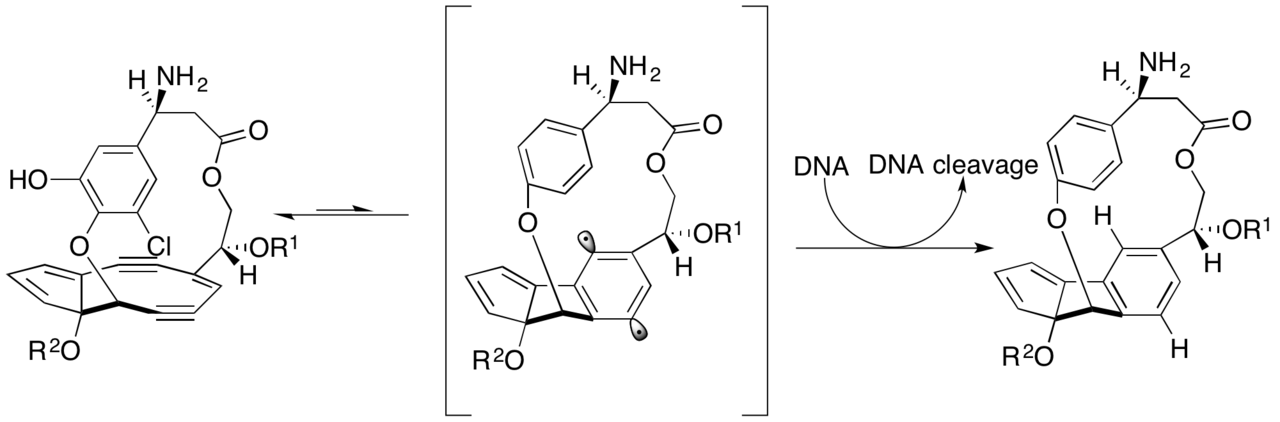
It causes double strand breaks in DNA from that central enediyene ring rearranging into a phenyl ring, which forms an extremely reactive benzenoid diradical, which cleaves both strands of DNA (a Bergman cyclization).
Mechanism below
This class of compounds is extremely potent, killing cultured cancer cells in low picomolar quantities.
This is impressive to me, because most things this toxic are catalysts; that is, they exert their damage in a cycle, that regenerates the damaging potential. Heavy metals for example can just repeatedly grab electrons and then give them away to water molecules, creating reactive oxygen species that damage biomolecules. Similar are enzymatic toxins like ricin, that slice off one RNA base on a ribosome, disabling them from translating proteins.
These enediyenes are different. They are stoichiometric toxins. The majority of toxins are like this, where their toxicity comes from the formation of a chemical bond that breaks something inside of a cell. Cyanide binds iron in blood cells and mitochondria so makes you unable to make ATP. Organophosphates bind to acetylcholinesterase and prevent acetylcholine hydrolysis. Alkylating agents like mustard gas simply react with proteins that have nucleophilic hot spots, like the sulfur in cystienes.
The enediyene ring opening can only occur once, so at best one molecule of lidamycin could cut one duplex of DNA.
To have such a high potency (think three orders of magnitude more potent than common chemotherapeutics) while not being catalytic (or doing something like activating a hormone receptor potently like dioxin) boggles my mind.
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,457
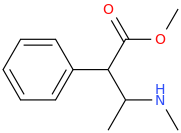
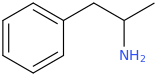
SATISFECHO
1-phenyl-1-carbomethoxy-2-methylaminopropane
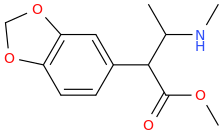
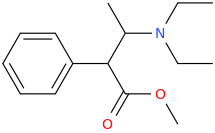
MUY_SATISFECHO
1-(3,4-methylenedioxyphenyl)-1-carbomethoxy-2-methylaminopropane
Just Can't Figure Out How The 2nd One Would Be Made. Also_Tapered?
A Penny For Your Thoughts?
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,457

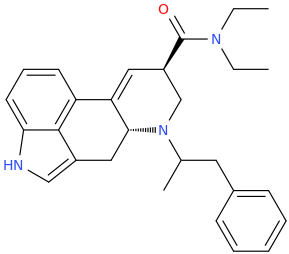
LSD-AMP
(6aR,9R)-N,N-diethyl-7-(1-methyl-2-phenylethyl)-4,6,6a,7,8,9-hexahydroindolo-[4,3-fg]-quinoline-9-carboxamide
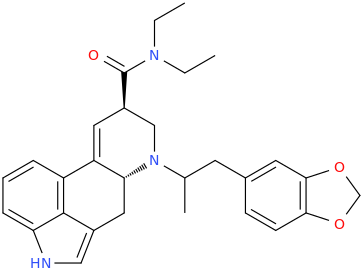
LSD-X
(6aR,9R)-N,N-diethyl-7-(1-methyl-2-(3,4-methylenedioxyphenyl)ethyl)-4,6,6a,7,8,9-hexahydroindolo-[4,3-fg]-quinoline-9-carboxamide
from nor-LSD
Last edited:
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,457
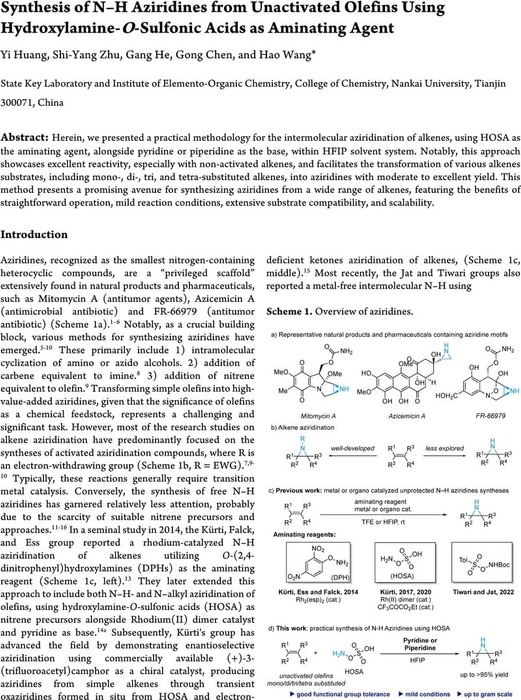
Synthesis of N–H Aziridines from Unactivated Olefins Using Hydroxylamine-O-Sulfonic Acids as Aminating Agent
Herein, we presented a practical methodology for the intermolecular aziridination of alkenes, using HOSA as the aminating agent, alongside pyridine or piperidine as the base, within HFIP solvent system. Notably, this approach showcases excellent reactivity, especially with non-activated alkenes...
I'm thinking once you have the N-H aziridine from the arene precursor, it could be opened somehow.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,457
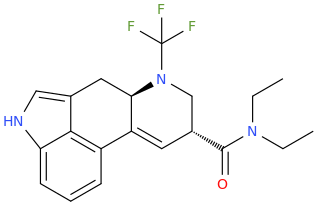
N-TFM-LSD
(6aR,9R)-N,N-diethyl-7-(trifluoromethyl)-4,6,6a,7,8,9-hexahydroindolo-%5b4,3-fg%5d-quinoline-9-carboxamide
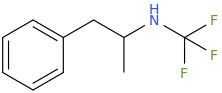
N-TFM-AMP
1-phenyl-2-(trifluoromethylamino)-propane
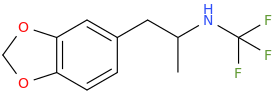
N-TFM-MDA
1-(3,4-methylenedioxyphenyl)-2-(trifluoromethylamino)-propane
Last edited: