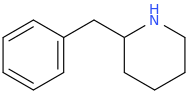
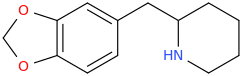
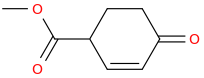
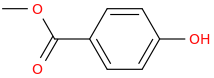
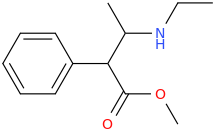
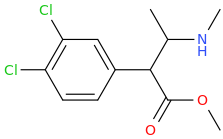
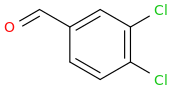
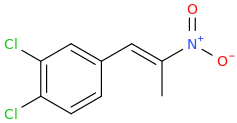
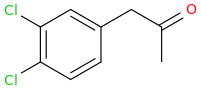
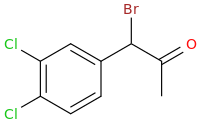
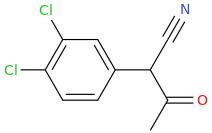
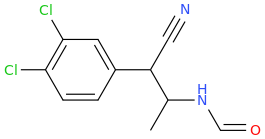
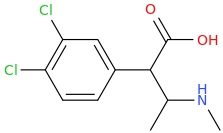
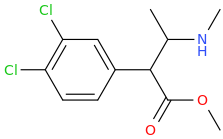
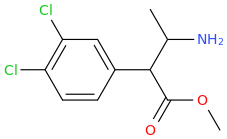
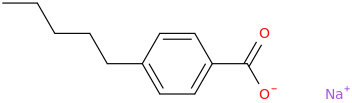
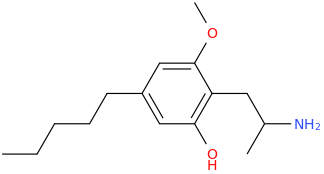
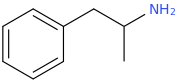
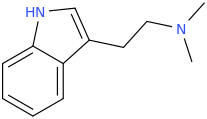
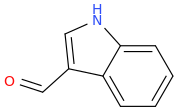
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,459
On The Quest For Better Antibiotics: This Fairly Recent Article Indicates That The Major Component Of Grapefruit Essential Oil (EO) Is Limonene. The Antimicrobial Effects Of Various Chemical Compounds Found In The EO Of Grapefruit Are Discussed. This EO Has Also Been Shown To Potentiate Certain Drugs.

 www.ncbi.nlm.nih.gov
www.ncbi.nlm.nih.gov

Chemical Composition, Antimicrobial, Antioxidant, and Antiproliferative Properties of Grapefruit Essential Oil Prepared by Molecular Distillation
Grapefruit essential oil has been proven to have wide range of bioactivities. However, bioactivity of its molecular distillate has not been well studied. In this study, a light phase oil was obtained by molecular distillation from cold-pressed grapefruit ...