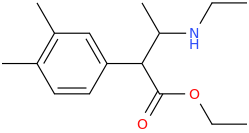
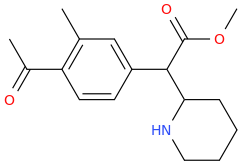
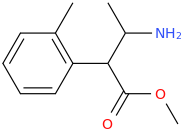
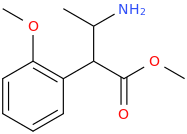
AlsoTapered
Bluelighter
- Joined
- Apr 1, 2023
- Messages
- 3,076
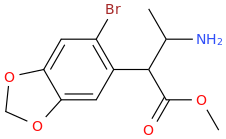
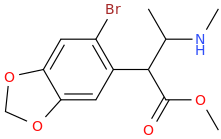
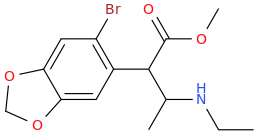
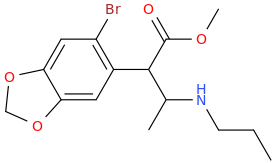
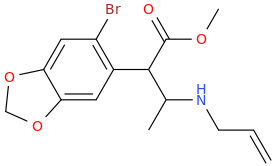
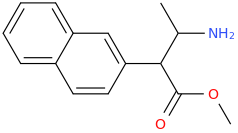
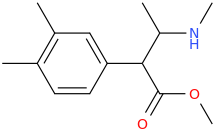
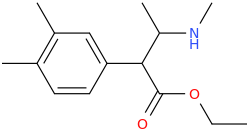
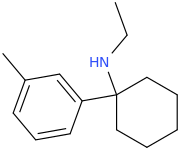
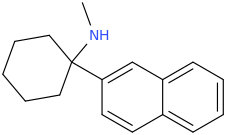
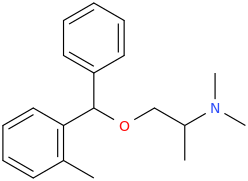
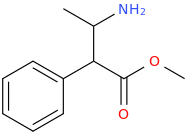
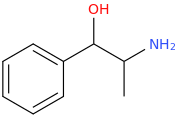
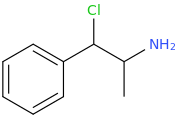
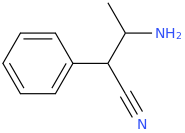
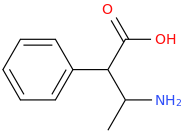
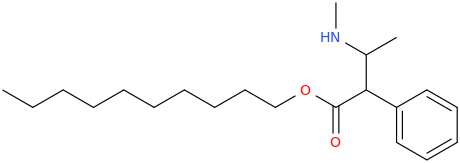
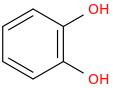
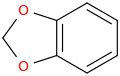
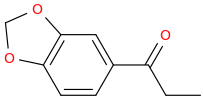
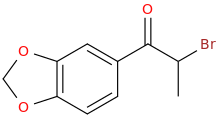
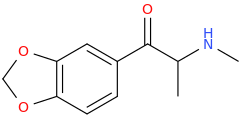
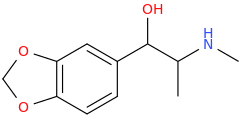
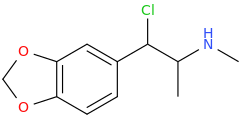
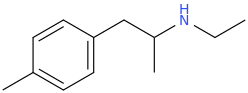
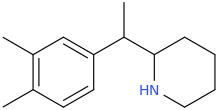
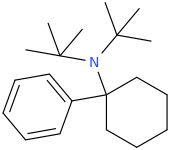
I'm not quite sure hw to put this, but show me these compounds in PubChem.
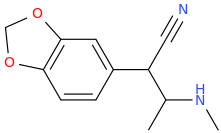
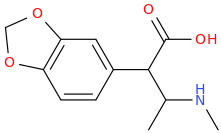
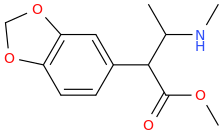
Aminorex and ring-substituted aminorex derivatives exist as zwitterions. That's why the subjective effects of 3,4-methylendioxiy aminorex is MORE active than MDA or MDMA and it longer acting and metabolism is predicable.
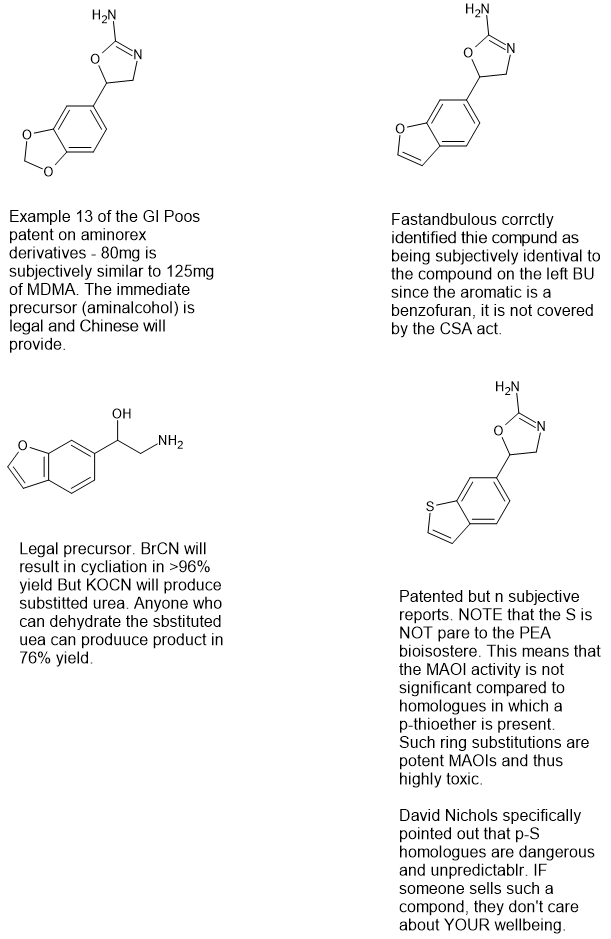
Example 13 of the original George Ireland Poos 1963 patent descries it in detail.
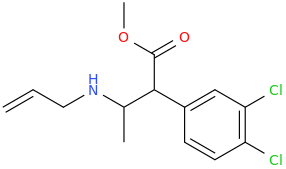
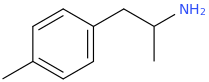
If I may suggest, I think people would appreciate someone detailing why the ring-substituted 4MAR derivatives are a LOT more toxic han the simple PEAs.
Even before we made example 13, I E-mailed David Nichols and his response was BRIEF. He said 'don't make the alpha methyl derivatives, they are really toxic.
Why? Because guanidine derivatives cause severe hypertension.
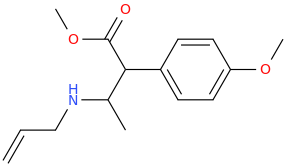
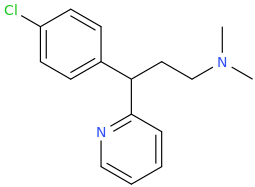
Fast and Bulbous suggested a homologue, we made it, it's like MDMA on steroids.
We studied the QSAR of the aminorex series and found:
-5HT2a affinity is not a parctical target i.e. one cannot produce 2,5-dimthoxy-4-<(pseudo)halogen> for psychedelic activity.
-4-methyl-aminorex derivatives have significant 5HT2b activity. Dr. David Nichols specifically suggested NOT tp test it or ring-substituted examples.
p-(pseudo)halogen examples ARE more potent BUT duration of action is VERY long.
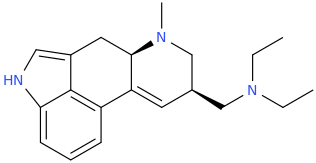
Replacing the aromatic with a benzofuran or a benzothiophene are as active as the ring-substituted benzene examples. Be clear, Nichols knows the law which is why an O or an S it part of the aromatic system - they are NOT substituted enzene rings containing O or Substituents.
BTW read PiHKAL. Shlgins recognized the key moities of ring-substitution.
Aminorex and ring-substituted aminorex derivatives exist as zwitterions. That's why the subjective effects of 3,4-methylendioxiy aminorex is MORE active than MDA or MDMA and it longer acting and metabolism is predicable.
Example 13 of the original George Ireland Poos 1963 patent descries it in detail.
If I may suggest, I think people would appreciate someone detailing why the ring-substituted 4MAR derivatives are a LOT more toxic han the simple PEAs.
Even before we made example 13, I E-mailed David Nichols and his response was BRIEF. He said 'don't make the alpha methyl derivatives, they are really toxic.
Why? Because guanidine derivatives cause severe hypertension.
Fast and Bulbous suggested a homologue, we made it, it's like MDMA on steroids.

We studied the QSAR of the aminorex series and found:
-5HT2a affinity is not a parctical target i.e. one cannot produce 2,5-dimthoxy-4-<(pseudo)halogen> for psychedelic activity.
-4-methyl-aminorex derivatives have significant 5HT2b activity. Dr. David Nichols specifically suggested NOT tp test it or ring-substituted examples.
p-(pseudo)halogen examples ARE more potent BUT duration of action is VERY long.
Replacing the aromatic with a benzofuran or a benzothiophene are as active as the ring-substituted benzene examples. Be clear, Nichols knows the law which is why an O or an S it part of the aromatic system - they are NOT substituted enzene rings containing O or Substituents.
BTW read PiHKAL. Shlgins recognized the key moities of ring-substitution.