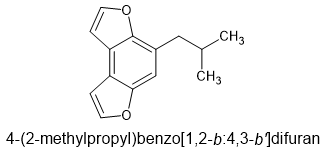
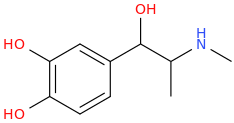
Fertile
Bluelighter
- Joined
- Mar 31, 2022
- Messages
- 1,627
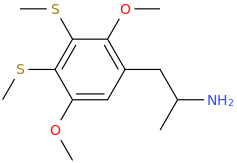
DIMMA
1-(2,5-dimethoxy-3,4-di(methylthio)phenyl)-2-aminopropane
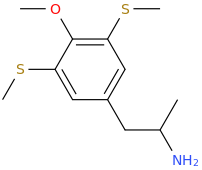
CYCLOBLACKOPS
1-(4-methoxy-3,5-di(methylthio)phenyl)-2-aminopropane
Please check Pubchem before claiming 'discovery' - these are already listed.