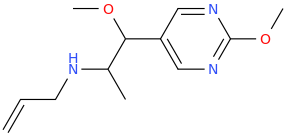
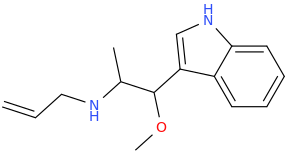
-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Ketamine salts solubility
- Thread starter fastandbulbous
- Start date
- Status
- Not open for further replies.
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,466
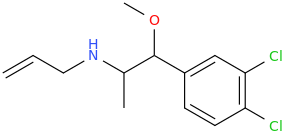
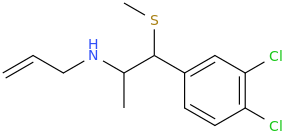
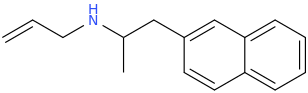
But What If I Never Have To Go To Sleep Again In Particular As Long As Sleep Deprivation Psychosis Doesn't Develop Say By Some Long Chance The Aromatic N-Allyl Promotes Just The Right Frequency To Calm The Mind Just Enough. This Stuff Is Not All Turbo Tweaker Super Power Focusing Like The Old Kind Was. And The Nature Favored Aromatic Addition Takes Away That Nasty Corrosive Drip Which In A Matter Of A Day Or Two Left Me With Oral And Sometimes Nasal Corrosion. In Fact, This Stuff Makes Your Nose Drip, Which Gives It A Little More Push! After A While It Is Almost Day Dreamer Material, With Warm Pastel Morning Color Enhancement. And I Am Getting Used To The Constant Pupil Dilation. The Cure For Sleep Without Psychosis? Now That Would Have Me Applying For A Nobel Prize.
Feretile
Bluelighter
- Joined
- Feb 2, 2022
- Messages
- 361
Some years ago I happened upon a new class of kappa ligands. As people may know, in many cases (such as U-47700) the difference between a kappa ligand and a mu ligand is a single methylene spacer. I've spent AGES looking for the paper with no luck.... but bless me, if I did not find the mu homologue of the compound, to whit:
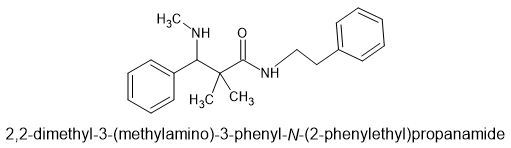
2,2-dimethyl-3-(methylamino)-3-phenyl-N-(2-phenylethyl)propanamide
As those who have read my paper on mu agonists, you will see 4 of the 5 key moieties and a biosteric minimum. I suggest that a p-Br (like BDPC) on the alpha benzene (closer to the amine) would increase potency as the compound is 14 methylenes long, not 15. The researchers did make the m-OH and made the alkyl chain an extra carbon long, but antagonist it was. The above is, to the best of my knowledge, an agonist.
Bioorganic & Medicinal Chemistry Letters
Martin P. Allen, James F.Blake, Dianne K.Bryce, Mary E. Haggan, Spiros Liras, Stafford McLean & Barb E.Segelstein
https://doi.org/10.1016/S0960-894X(00)00034-2
Received 1 October 1999, Accepted 4 January 2000, Available online 8 March 2000.
Design, synthesis and biological evaluation of 3-amino-3-phenylpropionamide derivatives as novel μ opioid receptor ligands
Steely eyed viewers may suggest that an -OH on the benzylic carbon of the beta aromatic MAY increase affinity. Then of course the mystery of why the active compounds are ALL secondary amines suggests that the secondaries are the more potent. The compound is also 14 methylene's in length and so their is an argument to add a p-Br (or p-Me) to the alpha benzene (the one closer to the amine moiety.
I do not think we can expect massive activity, but maybe 60x M is not unreasonable.
Oh, and on a point of secondary amines. the -CH3 of the amines forms a hydrogen-bond with the =O so you consider there to be an extra rung.

2,2-dimethyl-3-(methylamino)-3-phenyl-N-(2-phenylethyl)propanamide
As those who have read my paper on mu agonists, you will see 4 of the 5 key moieties and a biosteric minimum. I suggest that a p-Br (like BDPC) on the alpha benzene (closer to the amine) would increase potency as the compound is 14 methylenes long, not 15. The researchers did make the m-OH and made the alkyl chain an extra carbon long, but antagonist it was. The above is, to the best of my knowledge, an agonist.
Bioorganic & Medicinal Chemistry Letters
Martin P. Allen, James F.Blake, Dianne K.Bryce, Mary E. Haggan, Spiros Liras, Stafford McLean & Barb E.Segelstein
https://doi.org/10.1016/S0960-894X(00)00034-2
Received 1 October 1999, Accepted 4 January 2000, Available online 8 March 2000.
Design, synthesis and biological evaluation of 3-amino-3-phenylpropionamide derivatives as novel μ opioid receptor ligands
Steely eyed viewers may suggest that an -OH on the benzylic carbon of the beta aromatic MAY increase affinity. Then of course the mystery of why the active compounds are ALL secondary amines suggests that the secondaries are the more potent. The compound is also 14 methylene's in length and so their is an argument to add a p-Br (or p-Me) to the alpha benzene (the one closer to the amine moiety.
I do not think we can expect massive activity, but maybe 60x M is not unreasonable.
Oh, and on a point of secondary amines. the -CH3 of the amines forms a hydrogen-bond with the =O so you consider there to be an extra rung.
Feretile
Bluelighter
- Joined
- Feb 2, 2022
- Messages
- 361
Some years ago I happened upon a new class of kappa ligands. As people may know, in many cases (such as U-47700) the difference between a kappa ligand and a mu ligand is a single methylene spacer. I've spent AGES looking for the paper with no luck.... but bless me, if I did not find the mu homologue of the compound, to whit:

2,2-dimethyl-3-(methylamino)-3-phenyl-N-(2-phenylethyl)propanamide
As those who have read my paper on mu agonists, you will see 4 of the 5 key moieties and a biosteric minimum. I suggest that a p-Br (like BDPC) on the alpha benzene (closer to the amine) would increase potency as the compound is 14 methylenes long, not 15. The researchers did make the m-OH and made the alkyl chain an extra carbon long, but antagonist it was. The above is, to the best of my knowledge, an agonist.
Bioorganic & Medicinal Chemistry Letters
Martin P. Allen, James F.Blake, Dianne K.Bryce, Mary E. Haggan, Spiros Liras, Stafford McLean & Barb E.Segelstein
https://doi.org/10.1016/S0960-894X(00)00034-2
Received 1 October 1999, Accepted 4 January 2000, Available online 8 March 2000.
Design, synthesis and biological evaluation of 3-amino-3-phenylpropionamide derivatives as novel μ opioid receptor ligands
Steely eyed viewers may suggest that an -OH on the benzylic carbon of the beta aromatic MAY increase affinity. Then of course the mystery of why the active compounds are ALL secondary amines suggests that the secondaries are the more potent. The compound is also 14 methylene's in length and so their is an argument to add a p-Br (or p-Me) to the alpha benzene (the one closer to the amine moiety.
I do not think we can expect massive activity, but maybe 60x M is not unreasonable.
Oh, and on a point of secondary amines. the -CH3 of the amines forms a hydrogen-bond with the =O so you consider there to be an extra ring thus:
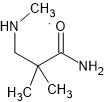
PS I would be just as keen to read about the antagonists (one's with meta -OH) and the kappa homologues (with extra methylene spacer.

2,2-dimethyl-3-(methylamino)-3-phenyl-N-(2-phenylethyl)propanamide
As those who have read my paper on mu agonists, you will see 4 of the 5 key moieties and a biosteric minimum. I suggest that a p-Br (like BDPC) on the alpha benzene (closer to the amine) would increase potency as the compound is 14 methylenes long, not 15. The researchers did make the m-OH and made the alkyl chain an extra carbon long, but antagonist it was. The above is, to the best of my knowledge, an agonist.
Bioorganic & Medicinal Chemistry Letters
Martin P. Allen, James F.Blake, Dianne K.Bryce, Mary E. Haggan, Spiros Liras, Stafford McLean & Barb E.Segelstein
https://doi.org/10.1016/S0960-894X(00)00034-2
Received 1 October 1999, Accepted 4 January 2000, Available online 8 March 2000.
Design, synthesis and biological evaluation of 3-amino-3-phenylpropionamide derivatives as novel μ opioid receptor ligands
Steely eyed viewers may suggest that an -OH on the benzylic carbon of the beta aromatic MAY increase affinity. Then of course the mystery of why the active compounds are ALL secondary amines suggests that the secondaries are the more potent. The compound is also 14 methylene's in length and so their is an argument to add a p-Br (or p-Me) to the alpha benzene (the one closer to the amine moiety.
I do not think we can expect massive activity, but maybe 60x M is not unreasonable.
Oh, and on a point of secondary amines. the -CH3 of the amines forms a hydrogen-bond with the =O so you consider there to be an extra ring thus:

PS I would be just as keen to read about the antagonists (one's with meta -OH) and the kappa homologues (with extra methylene spacer.
izo
Bluelighter
- Joined
- Mar 22, 2006
- Messages
- 4,165
49-CAA82-E-97-D5-40-C7-B212-1-BF6-E5-F8-F84-F hosted at ImgBB
Image 49-CAA82-E-97-D5-40-C7-B212-1-BF6-E5-F8-F84-F hosted in ImgBB
The Holy Quadruplty
Bluelighter
- Joined
- Feb 9, 2022
- Messages
- 660
You made those?
49-CAA82-E-97-D5-40-C7-B212-1-BF6-E5-F8-F84-F hosted at ImgBB
Image 49-CAA82-E-97-D5-40-C7-B212-1-BF6-E5-F8-F84-F hosted in ImgBBibb.co
Rectify
Bluelighter
- Joined
- Oct 20, 2008
- Messages
- 5,466
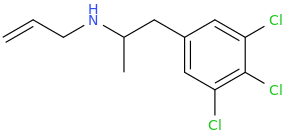
With Above Drug.

But If You Do Take This One, Which I Have Named For Jason Jones, Do Yourself A Rather Nicely Sized Favor And Cap It Up! Apparently Those 2 Aromatic Chlorines Are Not Entirely Pain Free If They Seep Into The Ear Canals Or Other Sensitive Membranes, Such As Those In And Around The Mouth.

But If You Do Take This One, Which I Have Named For Jason Jones, Do Yourself A Rather Nicely Sized Favor And Cap It Up! Apparently Those 2 Aromatic Chlorines Are Not Entirely Pain Free If They Seep Into The Ear Canals Or Other Sensitive Membranes, Such As Those In And Around The Mouth.
Last edited:
- Status
- Not open for further replies.