Feretile
Bluelighter
- Joined
- Feb 2, 2022
- Messages
- 361
Some years ago, while I was researching NMDA antagonists, I systematically went through all of the Parke-Davis patents on PCP US 3097136, all patents that cited it and all related patents. That eventually led to US 3254124, the original ketamine patent.
Now, what one discovers when browsing patents is that they almost all cover multiple compounds. The ketamine patent covers o substitutions of hydrogen, halogen, hydroxy, methyl and methoxy and dozens of different amine substitutions for the simple reason that if you don't cover all possible analogues, another company will quickly issue their own patent covering them.
What they did next was a surprise. They issued a patent for just 1 compound namely 2-chloro-5-methoxy ketamine. I do not know if it has a huge advantage, one assumes so. I can only imagine that since it's opioid activity is much greater, maybe sub-anesthetic doses were superior for treating pain. After all, I think K was licenced purely as an anesthetic.
Their research team then went on to find out if the optional =O could be replaced with a methyl moiety. As it turned out, not only was the compound a more active NMDA antagonist but it also had a much higher affinity for the mu opioid receptors & dopamine transport. Of course, such a modification introduces a second chiral centre, In the end, it turned out that while it was much more active, it's desired effects were no better and it proved to be more euphoric in animal models (or at least they would choose the drug over food for much longer - in fact I think they starved to death(.
This was unexpected because such trials were not common back in the 1970s. I'm sure people are aware of the famous study in which rats would choose cocaine over food until they starved BUT at the time, NMDA antagonists were not believed to cause dependence and/or addiction. I think we know better now.
Anyway, they also tried replacing the =O with a -CH3 in analogues that had a 2-thienyl aromatic rather than a (substituted benzene) and although the NMDA activity was even more pronounced, it did not seem to lead to self-destruction on the part of the test animals.
Lastly, I discovered a 2002 paper in the European Journal of Medicinal Chemistry (I had never even heard of the publication before then!) but they discovered that if the cyclohexane moiety with a 4-thiane, the LogP was significantly higher (3.36 ---> 4.78) AND conveniently, the S can undergo oxidation so the increased duration due to loss of the =O was offset.
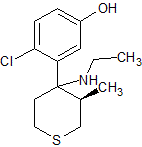
Forgive me if this is a little vague. I'm thinking back 11 years and am trying my best. If memory serves, the compound is: 1-[(3R,4R)-4-(2-chloro-5-methoxyphenyl)-3-methylthian-4-yl]piperidine
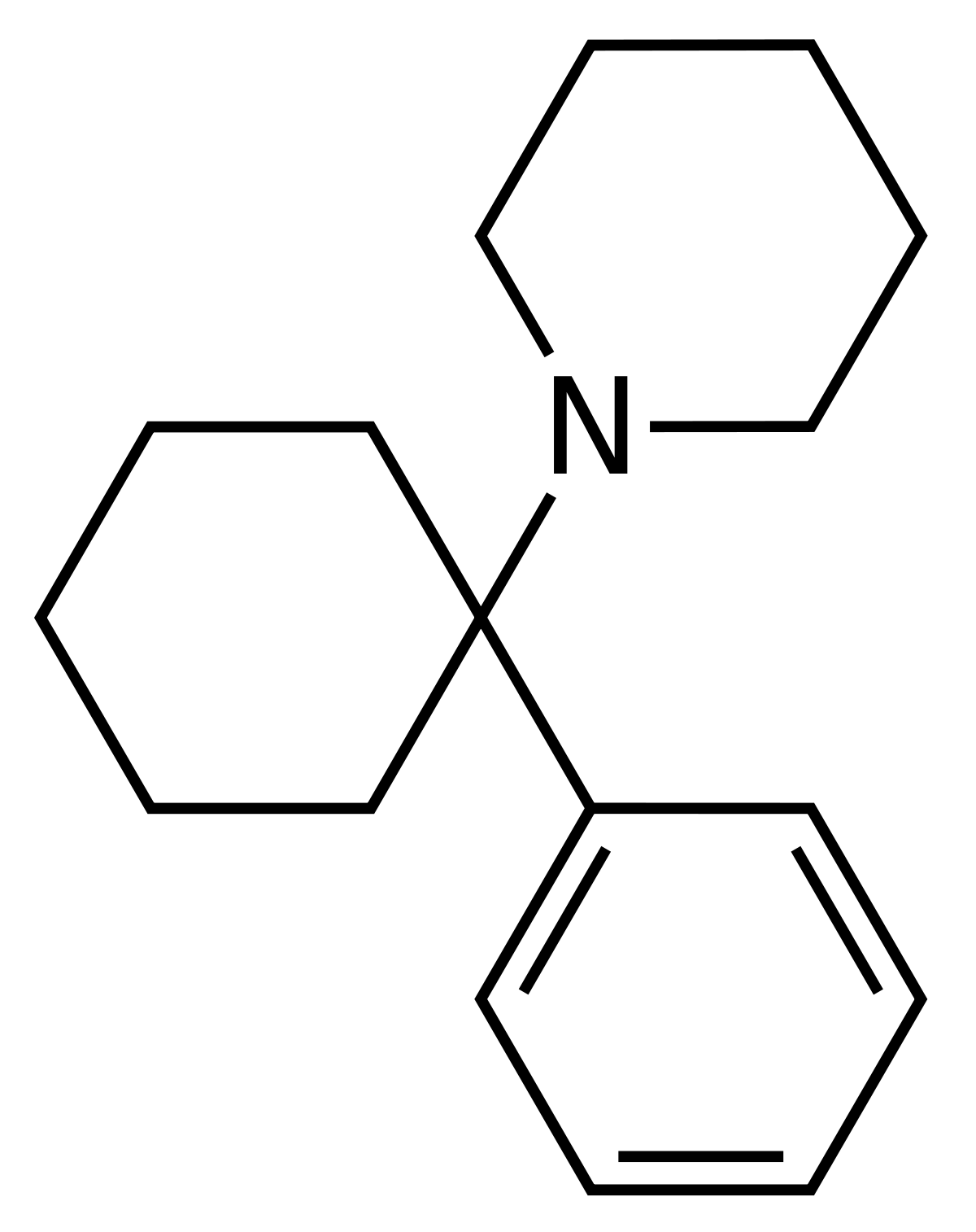
It seems that their are still researchers in this field but the cyclohexane->thiane may be of pracvtical use because most of the analogue laws I have read specify 'cvyclohexane;' and I presume that all of the cvompounds listed here: https://en.wikipedia.org/wiki/Arylcyclohexylamine would be as active if just a -CH2- is replaced by a -S- although I am no expert and strongly recommend that people check local laws AND have the compounds properly tested. We used to use HLS.
Now, what one discovers when browsing patents is that they almost all cover multiple compounds. The ketamine patent covers o substitutions of hydrogen, halogen, hydroxy, methyl and methoxy and dozens of different amine substitutions for the simple reason that if you don't cover all possible analogues, another company will quickly issue their own patent covering them.
What they did next was a surprise. They issued a patent for just 1 compound namely 2-chloro-5-methoxy ketamine. I do not know if it has a huge advantage, one assumes so. I can only imagine that since it's opioid activity is much greater, maybe sub-anesthetic doses were superior for treating pain. After all, I think K was licenced purely as an anesthetic.
Their research team then went on to find out if the optional =O could be replaced with a methyl moiety. As it turned out, not only was the compound a more active NMDA antagonist but it also had a much higher affinity for the mu opioid receptors & dopamine transport. Of course, such a modification introduces a second chiral centre, In the end, it turned out that while it was much more active, it's desired effects were no better and it proved to be more euphoric in animal models (or at least they would choose the drug over food for much longer - in fact I think they starved to death(.
This was unexpected because such trials were not common back in the 1970s. I'm sure people are aware of the famous study in which rats would choose cocaine over food until they starved BUT at the time, NMDA antagonists were not believed to cause dependence and/or addiction. I think we know better now.
Anyway, they also tried replacing the =O with a -CH3 in analogues that had a 2-thienyl aromatic rather than a (substituted benzene) and although the NMDA activity was even more pronounced, it did not seem to lead to self-destruction on the part of the test animals.
Lastly, I discovered a 2002 paper in the European Journal of Medicinal Chemistry (I had never even heard of the publication before then!) but they discovered that if the cyclohexane moiety with a 4-thiane, the LogP was significantly higher (3.36 ---> 4.78) AND conveniently, the S can undergo oxidation so the increased duration due to loss of the =O was offset.
Forgive me if this is a little vague. I'm thinking back 11 years and am trying my best. If memory serves, the compound is: 1-[(3R,4R)-4-(2-chloro-5-methoxyphenyl)-3-methylthian-4-yl]piperidine
It seems that their are still researchers in this field but the cyclohexane->thiane may be of pracvtical use because most of the analogue laws I have read specify 'cvyclohexane;' and I presume that all of the cvompounds listed here: https://en.wikipedia.org/wiki/Arylcyclohexylamine would be as active if just a -CH2- is replaced by a -S- although I am no expert and strongly recommend that people check local laws AND have the compounds properly tested. We used to use HLS.