BTW Professor James T. Cook at Minneapolis University School of Medicine is the Shulgin or Nichols of the benzo world. A name to look for.
Image qh hosted in ImgBB

ibb.co
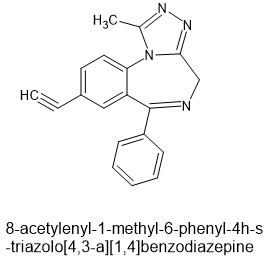
Above is the very first drug designed as a probe for the effects of alcohol. It is detailed in WO-03082832-A2. The 7-ethynyl stops all a2 & a3 affiinity affinity. The problem is like my efforts. half a dose does nothing, double up and you end up drunk in 10 minutes.
Image sch hosted in ImgBB

ibb.co
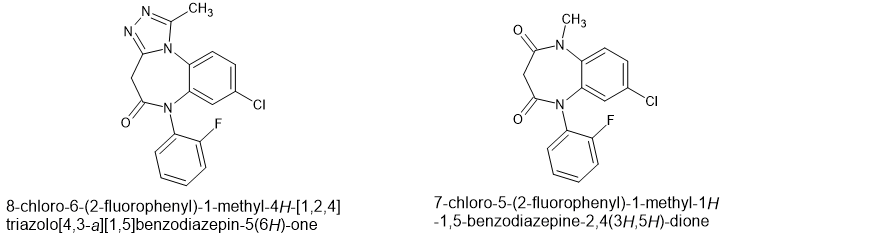
So then Cook introduced a triazolo ring which precludes a1 affinity and so the ONLY GABA subreceptor that the compound will bind to is the a5 and is it like ethanol? YES, it's JUST like alcohol except for the fast onset and long duration and slow decline. Still, 15mg gets you elephant's trunk,
Image tf hosted in ImgBB

ibb.co
Finally cook adds a '2F which increases potency by a factor of 5. Onset even faster, duration even longer, decline SLOW. Take on Friday night and you MAY make it out by Sunday afternoon.
But we independently proved that all of the negative effects of alcohol (amnesia, emotional lability, aggression, loss of executive control) are ALL mediated by a1 affinity (alcohol binds to a1 & a5). But a5 selective compounds also FEEL great.
https://ibb.co/XsKcfbL details it all.
BUT there is a problem - the fact that half a dose does nothing while 2 half doses leaves you asleep on the couch. My belief is that the a5 subunits of the b2 & b3 GABA receptors, although mild when taken alone, combine to produce a good, safe dose-response curve and so I need a 1,5-benzodiazepine.
Image 15 hosted in ImgBB

ibb.co
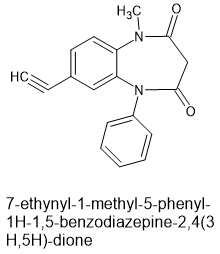
So here is the 1,5 homologue of the first compound Cook produced. EP-1925614-A1
Now having coresponded with professor Cook (a friend of Nutt- enough said?) I know he also produced the '2F derivative (x5 parent in potency) and the triazolo with '2F (some 15 x parent) which would be convenient since then a 2-bottle of wine level of trollied to the bladder, hammered, blasted, blitzed and mullered to the can in just 10 short minutes with the level of intoxication lasting 4-5 hours and then returns to abstemious state in another 4-5 makes it a highly enjoyable drug - one that 95% of adults are familiar with but few realize that a hangover is not a sure thing.Yes, you do enjoy your breakfast, but no dehydration, no illness, shakes, seizures or all of those bad things.
But presuming that 1mg of the dimer equals 1 UK unit of alcohol (10 mL of 100% ethanol) would mean that tax and duty would need to be agreed upon and adopted, licencing laws would need to be updates and the emergency services would need to recognise and appropriately react to an overdose. After all, be it ethanol or an ethanol mimic, do you want a driver on either to be using the road near you.
Then import and export, licencing of chemical designs and many, many other aspects would need to be dealt with.
Of course, one way is to simply put the stuff out there and let's see how the police control supply and distribution of something almost EVERY alcohol drinker would much prefer?