-
N&PD Moderators: Skorpio | thegreenhand
-
Neuroscience & Pharmacology Discussion Welcome Guest
Posting Rules Bluelight Rules Recent Journal Articles Chemistry Mega-Thread FREE Chemistry Databases! Self-Education Guide
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
SAR of meprobamate analogues
- Thread starter Solipsis
- Start date
I seem to recall an open-chain analogues of brallobarbital being involved in Brien Epstein's death. Carbromal, bromisoval, apronal and so on. Ah, now I look I see acecarbromal is amongst the class. There should be easily enough to provide a CHARMM training-set. It's a pity that an insufficient number of CPY-2D6 inducers are extant because glutethamide & codeine looked a risky but euphoric mixture. The isopropyl analogue of glutethimide would be the obvious one. Of course, with the UKs PDA in force, they would be illegal BUT it appears that aminoglutethimide is also an inducer. We learn a little new every day. If the structure is tolerant of haloalkyl and/or alkenyl groups then it may be possible to find something that alone is inactive but makes one's body convert codeine to morphine.
Last edited:
Not only did bromoureides share the overdose potential of barbiturates, but they also contained a pretty significant amount of bromine (1/3rd of Cabromal's mass came from that single bromine atom) so chronic use could lead to bromism.
I'm fascinated by this structure, though:
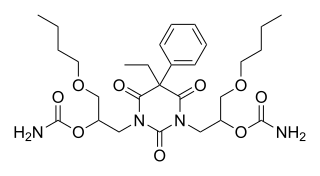
"Difebarbamate". Holy shit. Looks like one of those bizarre hybrid compounds that people on BL would come up with as a joke.
I'm fascinated by this structure, though:

"Difebarbamate". Holy shit. Looks like one of those bizarre hybrid compounds that people on BL would come up with as a joke.
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
Also, febarbamate, and the "combination drug" tetrabamate (Atrium, G Tril, Sevrium) = febarbamate, difebarbamate, and phenobarbital in combination.
Talk about OD potential.
Talk about OD potential.
bindingaffinity
Bluelighter
- Joined
- Apr 19, 2012
- Messages
- 150
Surprising that the reason they withdrew it was not for overdoses but for liver toxicity.
The picrotoxin/barbiturate site is promiscuous and a rather old, unfashionable target BUT the training set is HUGE. If you are prepared to let the software chug a way at minimum-energy states, you can find some compounds with a great deal of affinity. Among the huge set of amides, carbamates, haloalkyls and so forth, the 3D QSAR is not too fussy - most agents only conform to 2 moieties although it appears at least 4 exist. I don't think any new work has been done since the 1960s, or not much. Good point about the bromism, Hodor. I suppose that bromide salts are covered by the UK PDA! On the other hand, so is nutmeg... dumb law waiting to be circumvented by selling pro-drugs. If someone has to boil the powder for 1 hour to make the active, THEY are 'producing', not the seller. The UK RC will be back any day. I note amfonelic acid is being sold as an antibiotic for tropical fish... but no sellers being busted....
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
Hm...perhaps I need to start keeping tropical fish 
And the PDA is a non-law really from the very start. The only crime is being caught. Poorly thought out and downright moronic IMO.
I just hope against hope that come the next government, someone will have the common sense to repeal this abomination.
I find it a prototypical example of Theresa May and the way she is such a blunt-minded, pathetic, malignant, spineless little grub. She needs to be taken out, tied to a post and injected in the eyeballs with HF. It isn't as if she has any use for them anyway, stupid, bloated, noxious little grubs that spend all their time buried in the fabric of society, gnawing away at its integrity don't have much use for sight, it is plain. For May certainly has none to speak of.
I can just picture some native aboriginal tribesman hacking away at a rotting tree stump, and weaseling Theresa May out, wriggling on his palm, before calmly chomping down on It's body, sucking out the juices, before twisting off her head, flicking it to the dirt and crushing it underfoot as he walks off.
As for bromide salts...its to be hoped they won't get that stupid so as to start taking off after the likes of NaBr...got a kilo of the stuff in the kitchen, and a still-pot full of bromine. Not that I've ever taken the bromide. Although I suppose if ever there is a real emergency, it might come in useful in that respect, for seizure prophylaxis.
And the PDA is a non-law really from the very start. The only crime is being caught. Poorly thought out and downright moronic IMO.
I just hope against hope that come the next government, someone will have the common sense to repeal this abomination.
I find it a prototypical example of Theresa May and the way she is such a blunt-minded, pathetic, malignant, spineless little grub. She needs to be taken out, tied to a post and injected in the eyeballs with HF. It isn't as if she has any use for them anyway, stupid, bloated, noxious little grubs that spend all their time buried in the fabric of society, gnawing away at its integrity don't have much use for sight, it is plain. For May certainly has none to speak of.
I can just picture some native aboriginal tribesman hacking away at a rotting tree stump, and weaseling Theresa May out, wriggling on his palm, before calmly chomping down on It's body, sucking out the juices, before twisting off her head, flicking it to the dirt and crushing it underfoot as he walks off.
As for bromide salts...its to be hoped they won't get that stupid so as to start taking off after the likes of NaBr...got a kilo of the stuff in the kitchen, and a still-pot full of bromine. Not that I've ever taken the bromide. Although I suppose if ever there is a real emergency, it might come in useful in that respect, for seizure prophylaxis.
dumb law waiting to be circumvented by selling pro-drugs. If someone has to boil the powder for 1 hour to make the active, THEY are 'producing', not the seller. The UK RC will be back any day.
Are you talking about pro-drugs (i.e. something that gets converted into an active substance in-vivo) or precursors? If it's pro-drugs, I wish you the best of luck in convincing people that heroin and psilocybin are "non-psychoactive" because there's an (easily hydrolysed) ester in the way.
Making a substance that you can heat to convert it into an active form also limits your options in terms of what you can do with the structure. Sure you can boil off that carboxyl group or hydrolyse that amide, but there's a good chance your body's enzymes will be able to do the same, rendering the compound psychoactive in-vivo.
Also, being the one to *produce* the substance carries far, far, heavier penalties than merely being the user.
I note amfonelic acid is being sold as an antibiotic for tropical fish... but no sellers being busted....
Amfonelic acid is expensive a.f. though, and only used by a niche audience, so it might just fly under the government's radar. Plus, it actually *is* an antibiotic
I fear the days where people can buy grams of mephedrone and MXE at the local headshop for a few brit-bucks are gone, unless there's a freaking miracle.
Pro-drugs would be covered. A protecting group on a primary amine would be an example. A carbamate maybe? Benzylideneamine? Something that a swift pH change will shake off and not be toxic.... Vendor is therefore selling an inactive compound thus it is legal. I recall people selling GBL in about 1992-1993 (wow, I am so old).
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
Well wouldn't it first have to be proved that one knew it was psychoactive?
As for H, and other morphine esters, they aren't just prodrugs, they are active and have a distinct, and different pharmacology all of their own.
As for H, and other morphine esters, they aren't just prodrugs, they are active and have a distinct, and different pharmacology all of their own.
Solipsis
Bluelight Crew
- Joined
- Mar 12, 2007
- Messages
- 15,509
Not really, just that it is a pro-drug of a scheduled compound or a pro-drug of something analogous to a scheduled compound?
As for the seizure prophylaxis: good thing then it's not LiBr (not a 1337 way of abbreviating librium)
(not a 1337 way of abbreviating librium)
As for my original question: I am not really well versed in barbs, but I will check out the SAR patterns of very basic substitutions like the simple permutations of the first homologue series.
As for the seizure prophylaxis: good thing then it's not LiBr
As for my original question: I am not really well versed in barbs, but I will check out the SAR patterns of very basic substitutions like the simple permutations of the first homologue series.
Limpet_Chicken
Bluelighter
- Joined
- Oct 13, 2005
- Messages
- 6,323
Hm, suppose it could make it difficult in court PROVING it was psychoactive (from a UK POV)
Seizure prophylaxis? well I consider it there if there's an emergency, but my NaBr is just meant unless the shit really does hit the fan, for generating Br2 or HBr, if theres trouble, then yeah, I'd use it if I had to. Although I'm too used to seeing it fuming off acrid brown stinking fumes to view it as tasty, per se, or at least choking gas.
Seizure prophylaxis? well I consider it there if there's an emergency, but my NaBr is just meant unless the shit really does hit the fan, for generating Br2 or HBr, if theres trouble, then yeah, I'd use it if I had to. Although I'm too used to seeing it fuming off acrid brown stinking fumes to view it as tasty, per se, or at least choking gas.
sekio
Bluelight Crew
- Joined
- Sep 14, 2009
- Messages
- 21,994
Nope, as far as I know carbamic acid esters are kind of obsolete drugs, same as any of the barbiturate-type drugs.
Also, you don't have to post the same question on 3 different threads (2 of which haven't been active for years). I removed the other posts.
Also, you don't have to post the same question on 3 different threads (2 of which haven't been active for years). I removed the other posts.
