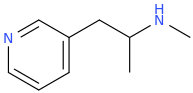
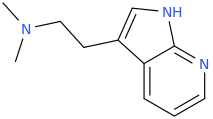
Those lone-pairs will give an interesting result. No p-OH can be formed so oxidation of the alphamethyl group although the compound will be less lipophilic. Compare pyrazolam - exit's body unmetabolized. The -N= prevents the liver enzymes from breaking it down and in spite of it's lower cLogP, 0.5-1.0mg does help anxiety while having little abuse potential. The nitro proved to have potent anti-depressant action (to bridge presentation to SSRIs working) while the ethynly is like all the good stuff in alcohol without the dark corners (NMDA obviously makes people violent). So ideal for alcohol detox. So all 3 have commercial use, but I can't afford to patent them. The synthesis is the patentable as are the confirmed (in rodent model) actions of the other 2. Same thing, not metabolized. That scaffold with EWGs, EDGs & just steric bulk (2-trifluoroethyl was interesting as a hypnotic that, again, had much less abuse potential. First into man....